A compartment model of VEGF distribution in humans in the presence of soluble VEGF receptor-1 acting as a ligand trap
- PMID: 19352513
- PMCID: PMC2663039
- DOI: 10.1371/journal.pone.0005108
A compartment model of VEGF distribution in humans in the presence of soluble VEGF receptor-1 acting as a ligand trap
Abstract
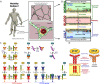
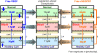
Vascular endothelial growth factor (VEGF), through its activation of cell surface receptor tyrosine kinases including VEGFR1 and VEGFR2, is a vital regulator of stimulatory and inhibitory processes that keep angiogenesis--new capillary growth from existing microvasculature--at a dynamic balance in normal physiology. Soluble VEGF receptor-1 (sVEGFR1)--a naturally-occurring truncated version of VEGFR1 lacking the transmembrane and intracellular signaling domains--has been postulated to exert inhibitory effects on angiogenic signaling via two mechanisms: direct sequestration of angiogenic ligands such as VEGF; or dominant-negative heterodimerization with surface VEGFRs. In pre-clinical studies, sVEGFR1 gene and protein therapy have demonstrated efficacy in inhibiting tumor angiogenesis; while in clinical studies, sVEGFR1 has shown utility as a diagnostic or prognostic marker in a widening array of angiogenesis-dependent diseases. Here we developed a novel computational multi-tissue model for recapitulating the dynamic systemic distributions of VEGF and sVEGFR1. Model features included: physiologically-based multi-scale compartmentalization of the human body; inter-compartmental macromolecular biotransport processes (vascular permeability, lymphatic drainage); and molecularly-detailed binding interactions between the ligand isoforms VEGF(121) and VEGF(165), signaling receptors VEGFR1 and VEGFR2, non-signaling co-receptor neuropilin-1 (NRP1), as well as sVEGFR1. The model was parameterized to represent a healthy human subject, whereupon we investigated the effects of sVEGFR1 on the distribution and activation of VEGF ligands and receptors. We assessed the healthy baseline stability of circulating VEGF and sVEGFR1 levels in plasma, as well as their reliability in indicating tissue-level angiogenic signaling potential. Unexpectedly, simulated results showed that sVEGFR1 - acting as a diffusible VEGF sink alone, i.e., without sVEGFR1-VEGFR heterodimerization--did not significantly lower interstitial VEGF, nor inhibit signaling potential in tissues. Additionally, the sensitivity of plasma VEGF and sVEGFR1 to physiological fluctuations in transport rates may partially account for the heterogeneity in clinical measurements of these circulating angiogenic markers, potentially hindering their diagnostic reliability for diseases.
Conflict of interest statement
Figures








References
-
- Yla-Herttuala S, Rissanen TT, Vajanto I, Hartikainen J. Vascular endothelial growth factors: Biology and current status of clinical applications in cardiovascular medicine. J Am Coll Cardiol. 2007;49(10):1015–1026. - PubMed
-
- Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407(6801):249–257. - PubMed
-
- Gustafsson T, Ameln H, Fischer H, Sundberg CJ, Timmons JA, et al. VEGF-A splice variants and related receptor expression in human skeletal muscle following submaximal exercise. J Appl Physiol. 2005;98(6):2137–2146. - PubMed
-
- Ng YS, Rohan R, Sunday ME, Demello DE, D'Amore PA. Differential expression of VEGF isoforms in mouse during development and in the adult. Dev Dyn. 2001;220(2):112–121. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

