Neurosphere formation is an independent predictor of clinical outcome in malignant glioma
- PMID: 19353526
- PMCID: PMC3177534
- DOI: 10.1002/stem.15
Neurosphere formation is an independent predictor of clinical outcome in malignant glioma
Abstract
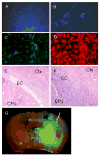
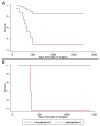
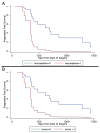
Renewable neurosphere formation in culture is a defining characteristic of certain brain tumor initiating cells. This retrospective study was designed to assess the relationship among neurosphere formation in cultured human glioma, tumorigenic capacity, and patient clinical outcome. Tumor samples were cultured in neurosphere conditions from 32 patients with glioma, including a subpopulation of 15 patients with primary glioblastoma. A subsample of renewable neurosphere cultures was xenografted into mouse brain to determine if they were tumorigenic. Our study shows that both renewable neurosphere formation and tumorigenic capacity are significantly associated with clinical outcome measures. Renewable neurosphere formation in cultured human glioma significantly predicted an increased hazard of patient death and more rapid tumor progression. These results pertained to both the full population of glioma and the subpopulation of primary glioblastoma. Similarly, there was a significant hazard of progression for patients whose glioma had tumorigenic capacity. Multivariate analysis demonstrated that neurosphere formation remained a significant predictor of clinical outcome independent of Ki67 proliferation index. In addition, multivariate analysis of neurosphere formation, tumor grade and patient age, demonstrated that neurosphere formation was a robust, independent predictor of glioma tumor progression. Although the lengthy duration of this assay may preclude direct clinical application, these results exemplify how neurosphere culture serves as a clinically relevant model for the study of malignant glioma. Furthermore, this study suggests that the ability to propagate brain tumor stem cells in vitro is associated with clinical outcome.
Conflict of interest statement
There are no potential conflicts of interest.
Figures
References
-
- Buckner JC, Brown PD, O’Neill BP, et al. Central nervous system tumors. Mayo Clin Proc. 2007;82:1271–1286. - PubMed
-
- Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. - PubMed
-
- Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–1710. - PubMed
-
- Galli R, Binda E, Orfanelli U, et al. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 2004;64:7011–7021. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

