Isolation and characterization of a small antiretroviral molecule affecting HIV-1 capsid morphology
- PMID: 19356241
- PMCID: PMC2670814
- DOI: 10.1186/1742-4690-6-34
Isolation and characterization of a small antiretroviral molecule affecting HIV-1 capsid morphology
Abstract
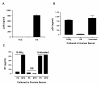
Background: Formation of an HIV-1 particle with a conical core structure is a prerequisite for the subsequent infectivity of the virus particle. We have previously described that glycineamide (G-NH2) when added to the culture medium of infected cells induces non-infectious HIV-1 particles with aberrant core structures.
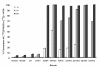
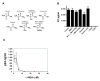
Results: Here we demonstrate that it is not G-NH2 itself but a metabolite thereof that displays antiviral activity. We show that conversion of G-NH2 to its antiviral metabolite is catalyzed by an enzyme present in bovine and porcine but surprisingly not in human serum. Structure determination by NMR suggested that the active G-NH2 metabolite was alpha-hydroxy-glycineamide (alpha-HGA). Chemically synthesized alpha-HGA inhibited HIV-1 replication to the same degree as G-NH2, unlike a number of other synthesized analogues of G-NH2 which had no effect on HIV-1 replication. Comparisons by capillary electrophoresis and HPLC of the metabolite with the chemically synthesized alpha-HGA further confirmed that the antiviral G-NH2-metabolite indeed was alpha-HGA.
Conclusion: alpha-HGA has an unusually simple structure and a novel mechanism of antiviral action. Thus, alpha-HGA could be a lead for new antiviral substances belonging to a new class of anti-HIV drugs, i.e. capsid assembly inhibitors.
Figures

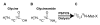

References
-
- Briggs JA, Simon MN, Gross I, Krausslich HG, Fuller SD, Vogt VM, Johnson MC. The stoichiometry of Gag protein in HIV-1. Nat Struct Mol Biol. 2004;11:672–675. - PubMed
-
- Lanman J, Lam TT, Barnes S, Sakalian M, Emmett MR, Marshall AG, Prevelige PE., Jr Identification of novel interactions in HIV-1 capsid protein assembly by high-resolution mass spectrometry. J Mol Biol. 2003;325:759–772. - PubMed
-
- Li S, Hill CP, Sundquist WI, Finch JT. Image reconstructions of helical assemblies of the HIV-1 CA protein. Nature. 2000;407:409–413. - PubMed
-
- Gamble TR, Vajdos FF, Yoo S, Worthylake DK, Houseweart M, Sundquist WI, Hill CP. Crystal structure of human cyclophilin A bound to the amino-terminal domain of HIV-1 capsid. Cell. 1996;87:1285–1294. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

