Rabies DNA vaccine: no impact of MHC class I and class II targeting sequences on immune response and protection against lethal challenge
- PMID: 19356616
- PMCID: PMC7115670
- DOI: 10.1016/j.vaccine.2009.01.128
Rabies DNA vaccine: no impact of MHC class I and class II targeting sequences on immune response and protection against lethal challenge
Abstract
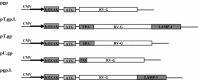
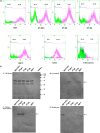
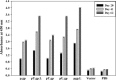
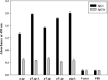
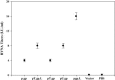
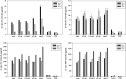
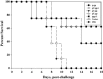
Rabies is progressive fatal encephalitis. WHO estimates 55,000 rabies deaths and more than 10 million PEP every year world-wide. A variety of cell-culture derived vaccines are available for prophylaxis against rabies. However, their high cost restricts their usage in developing countries, where such cases are most often encountered. This is driving the quest for newer vaccine formulations; DNA vaccines being most promising amongst them. Here, we explored strategies of antigen trafficking to various cellular compartments aiming at improving both humoral and cellular immunity. These strategies include use of signal sequences namely Tissue Plasminogen Activator (TPA), Ubiquitin (UQ) and Lysosomal-Associated Membrane Protein-1 (LAMP-1). TPA, LAMP-1 and their combination were aimed at enhancing the CD4(+) T cell and antibody response. In contrast, the UQ tag was utilized for enhancing CD8(+) response. The potency of modified DNA vaccines assessed by total antibody response, antibody isotypes, cytokine profile, neutralizing antibody titer and protection conferred against in vivo challenge; was enhanced in comparison to native unmodified vaccine, but the response elicited did not pertain to the type of target sequence and the directed arm of immunity. Interestingly, the DNA vaccines that had been designed to generate different type of immune responses yielded in effect similar response. In conclusion, our data indicate that the directing target sequence is not the exclusive deciding factor for type and extent of immune response elicited and emphasizes on the antigen dependence of immune enhancement strategies.
Figures
Similar articles
-
Addition of C3d-P28 adjuvant to a rabies DNA vaccine encoding the G5 linear epitope enhances the humoral immune response and confers protection.Vaccine. 2018 Jan 4;36(2):292-298. doi: 10.1016/j.vaccine.2017.11.047. Epub 2017 Nov 27. Vaccine. 2018. PMID: 29191739
-
Alum adjuvanted rabies DNA vaccine confers 80% protection against lethal 50 LD50 rabies challenge virus standard strain.Mol Immunol. 2017 May;85:166-173. doi: 10.1016/j.molimm.2017.02.011. Epub 2017 Mar 6. Mol Immunol. 2017. PMID: 28267643
-
Hantavirus Gc induces long-term immune protection via LAMP-targeting DNA vaccine strategy.Antiviral Res. 2018 Feb;150:174-182. doi: 10.1016/j.antiviral.2017.12.011. Epub 2017 Dec 20. Antiviral Res. 2018. PMID: 29273568
-
Canine adenovirus based rabies vaccines.Dev Biol (Basel). 2008;131:467-76. Dev Biol (Basel). 2008. PMID: 18634509 Review.
-
Human monoclonal antibody and vaccine approaches to prevent human rabies.Curr Top Microbiol Immunol. 2008;317:67-101. doi: 10.1007/978-3-540-72146-8_3. Curr Top Microbiol Immunol. 2008. PMID: 17990790 Review.
Cited by
-
Fusion to Flaviviral Leader Peptide Targets HIV-1 Reverse Transcriptase for Secretion and Reduces Its Enzymatic Activity and Ability to Induce Oxidative Stress but Has No Major Effects on Its Immunogenic Performance in DNA-Immunized Mice.J Immunol Res. 2017;2017:7407136. doi: 10.1155/2017/7407136. Epub 2017 Jun 22. J Immunol Res. 2017. PMID: 28717654 Free PMC article.
-
Enhancement of immunogenicity and efficacy of a plasmid DNA rabies vaccine by nanoformulation with a fourth-generation amine-terminated poly(ether imine) dendrimer.Int J Nanomedicine. 2014 Jan 28;9:627-34. doi: 10.2147/IJN.S53415. eCollection 2014. Int J Nanomedicine. 2014. PMID: 24501540 Free PMC article.
-
Rabies Control and Treatment: From Prophylaxis to Strategies with Curative Potential.Viruses. 2016 Oct 28;8(11):279. doi: 10.3390/v8110279. Viruses. 2016. PMID: 27801824 Free PMC article. Review.
-
Developments in Rabies Vaccines: The Path Traversed from Pasteur to the Modern Era of Immunization.Vaccines (Basel). 2023 Mar 29;11(4):756. doi: 10.3390/vaccines11040756. Vaccines (Basel). 2023. PMID: 37112668 Free PMC article. Review.
-
The future of human DNA vaccines.J Biotechnol. 2012 Dec 31;162(2-3):171-82. doi: 10.1016/j.jbiotec.2012.08.012. Epub 2012 Sep 7. J Biotechnol. 2012. PMID: 22981627 Free PMC article. Review.
References
-
- Rupprecht C., Hanlon C.A., Hemachuda T. Rabies re-examined. Lancet Infect Dis. 2002;2(6):327–343. - PubMed
-
- World survey of rabies: No. 32 for the year 1996. Geneva, World Health Organization; 1998 (WHO/EMC/ZDI/98.4).
-
- Smith J.S., Seidel H.D. Rabies: a new look at an old disease. Prog Med Virol. 1993;40:82–106. - PubMed
-
- Chattergoon M., Bare J., Weiner D.B. Genetic immunization: a new era in vaccines and immunotherapy. FASEB J. 1997;11(10):753–763. - PubMed
-
- Donnely J., Ulmer J.B., Shiver J.W., Liu M.A. DNA vaccine. Annu Rev Immunol. 1997;15:617–648. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

