Role of B-Raf(V600E) in differentiated thyroid cancer and preclinical validation of compounds against B-Raf(V600E)
- PMID: 19356676
- PMCID: PMC3814130
- DOI: 10.1016/j.bbcan.2009.01.003
Role of B-Raf(V600E) in differentiated thyroid cancer and preclinical validation of compounds against B-Raf(V600E)
Abstract
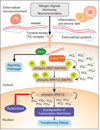
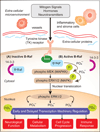
B-Raf(V600E), an oncogenic protein kinase, is the most frequent genetic alteration in papillary thyroid carcinomas (PTC). PTC represents 80-90% of all thyroid cancers and over the past five years, more than 200 manuscripts have been published about the relationship between "B-Raf(V600E) and thyroid cancer". B-Raf(V600E) genetically arises from a transversion point mutation (valine-to-glutamate substitution at amino acid residue-600, V600E) and leads to over activation of the mitogen-activated protein kinases (MAPK) signaling pathway. The MAPK pathway is essential for transmitting proliferation signals generated by cell surface receptors and cytoplasmic signaling elements to the nucleus. In many cancers, including thyroid cancer, B-Raf(V600E) appears to play a crucial role in cell proliferation, survival and de-differentiation. In thyroid cancer, the V600E mutation occurs with greater frequently in aggressive subtypes of PTC, and in individuals that present at advanced stages of disease with extra-thyroidal extension and/or lymph node metastases. B-Raf(V600E) is considered a marker of aggressive disease in both PTC (>1 cm) and micro-PTC (</=1 cm), and interestingly, is associated with both loss of I-131 avidity and PTC recurrence. Though treatment of patients with thyroid cancer is usually successful and most patients are rendered disease-free, to date there are no effective therapies for patients with invasive, non-radioiodine sensitive tumors or metastatic disease. In this article we will review the relation between B-Raf(V600E) and PTC, as well as both non-selective and selective pharmacological agents currently under investigation for treatment of B-Raf(V600E) positive PTC.
Figures
References
-
- S.E.a.E.R.S. Program. 2007 www.seer.cancer.gov.
-
- NCCN. Practice guidelines in Oncology. 2007 www.nccn.org.
-
- Kondo T, Ezzat S, Asa SL. Pathogenetic mechanisms in thyroid follicular-cell neoplasia. Nat Rev Cancer. 2006;6:292–306. - PubMed
-
- Ciampi R, Nikiforov YE. RET/PTC rearrangements and BRAF mutations in thyroid tumorigenesis. Endocrinology. 2007;148:936–941. - PubMed
-
- Santoro M, Melillo RM, Carlomagno F, Fusco A, Vecchio G. Molecular mechanisms of RET activation in human cancer. Ann N Y Acad Sci. 2002;963:116–121. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

