Endothelial actin and cell stiffness is modulated by substrate stiffness in 2D and 3D
- PMID: 19356760
- PMCID: PMC2893018
- DOI: 10.1016/j.jbiomech.2009.02.012
Endothelial actin and cell stiffness is modulated by substrate stiffness in 2D and 3D
Abstract
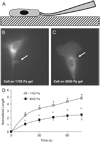
There is a growing appreciation of the profound effects that passive mechanical properties, especially the stiffness of the local environment, can have on cellular functions. Many experiments are conducted in a 2D geometry (i.e., cells grown on top of substrates of varying stiffness), which is a simplification of the 3D environment often experienced by cells in vivo. To determine how matrix dimensionality might modulate the effect of matrix stiffness on actin and cell stiffness, endothelial cells were cultured on top of and within substrates of various stiffnesses. Endothelial cells were cultured within compliant (1.0-1.5mg/ml, 124+/-8 to 202+/-27Pa) and stiff (3.0mg/ml, 502+/-48Pa) type-I collagen gels. Cells elongated and formed microvascular-like networks in both sets of gels as seen in previous studies. Cells in stiffer gels exhibited more pronounced stress fibers and approximately 1.5-fold greater staining for actin. As actin is a major determinant of a cell's mechanical properties, we hypothesized that cells in stiff gels will themselves be stiffer. To test this hypothesis, cells were isolated from the gels and their stiffness was assessed using micropipette aspiration. Cells isolated from relatively compliant gels were 1.9-fold more compliant than cells isolated from relatively stiff gels (p<0.05). Similarly, cells cultured on top of 1700Pa polyacrylamide gels were 2.0-fold more compliant that those cultured on 9000Pa (p<0.05). These data demonstrate that extracellular substrate stiffness regulates endothelial stiffness in both three- and two-dimensional environments, though the range of stiffnesses that cells respond to vary significantly in different environments.
Conflict of interest statement
No conflicts.
Figures


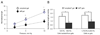
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

