Acquisition of polyfunctionality by Epstein-Barr virus-specific CD8+ T cells correlates with increased resistance to galectin-1-mediated suppression
- PMID: 19357166
- PMCID: PMC2687380
- DOI: 10.1128/JVI.00239-09
Acquisition of polyfunctionality by Epstein-Barr virus-specific CD8+ T cells correlates with increased resistance to galectin-1-mediated suppression
Abstract
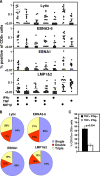
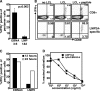
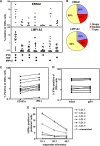
Latent membrane antigen 1 and -2 (LMP-1/2)-specific CD8(+) T cells from newly diagnosed and relapsed Hodgkin's lymphoma (HL) patients display a selective functional impairment. In contrast, CD8(+) T cells specific for Epstein-Barr virus (EBV) nuclear proteins and lytic antigens retain normal T-cell function. Reversion to a dysfunctional phenotype of LMP-1/2-specific T cells is coincident with the regression of HL. To delineate the potential basis for this differential susceptibility for the loss of function, we have carried out a comprehensive functional analysis of EBV-specific T cells using ex vivo multiparametric flow cytometry in combination with assessment of antigen-driven proliferative potential. This analysis revealed that LMP-1/2-specific T cells from healthy virus carriers display a deficient polyfunctional profile compared to that of T cells specific for epitopes derived from EBV nuclear proteins and lytic antigens. Furthermore, LMP-specific T-cells are highly susceptible to galectin-1-mediated immunosuppression and are less likely to degranulate following exposure to cognate peptide epitopes and poorly recognized endogenously processed epitopes from virus-infected B cells. More importantly, ex vivo stimulation of these T cells with an adenoviral vector encoding multiple minimal CD8(+) T-cell epitopes as a polyepitope, in combination with a gammaC cytokine, interleukin-2, restored polyfunctionality and shielded these cells from the inhibitory effects of galectin-1.
Figures

References
-
- Almeida, J. R., D. A. Price, L. Papagno, Z. A. Arkoub, D. Sauce, E. Bornstein, T. E. Asher, A. Samri, A. Schnuriger, I. Theodorou, D. Costagliola, C. Rouzioux, H. Agut, A. G. Marcelin, D. Douek, B. Autran, and V. Appay. 2007. Superior control of HIV-1 replication by CD8+ T cells is reflected by their avidity, polyfunctionality, and clonal turnover. J. Exp. Med. 2042473-2485. - PMC - PubMed
-
- Badr, G., N. Bedard, M. S. Abdel-Hakeem, L. Trautmann, B. Willems, J. P. Villeneuve, E. K. Haddad, R. P. Sekaly, J. Bruneau, and N. H. Shoukry. 2008. Early interferon therapy for hepatitis C virus infection rescues polyfunctional, long-lived CD8+ memory T cells. J. Virol. 8210017-10031. - PMC - PubMed
-
- Gandhi, M. K., E. Lambley, J. Duraiswamy, U. Dua, C. Smith, S. Elliott, D. Gill, P. Marlton, J. Seymour, and R. Khanna. 2006. Expression of LAG-3 by tumor-infiltrating lymphocytes is coincident with the suppression of latent membrane antigen-specific CD8+ T-cell function in Hodgkin lymphoma patients. Blood 1082280-2289. - PubMed
-
- Guppy, A. E., E. Rawlings, J. A. Madrigal, P. L. Amlot, and L. D. Barber. 2007. A quantitative assay for Epstein-Barr virus-specific immunity shows interferon-gamma producing CD8+ T cells increase during immunosuppression reduction to treat posttransplant lymphoproliferative disease. Transplantation 841534-1539. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

