Characterization of Imjin virus, a newly isolated hantavirus from the Ussuri white-toothed shrew (Crocidura lasiura)
- PMID: 19357167
- PMCID: PMC2687366
- DOI: 10.1128/JVI.00371-09
Characterization of Imjin virus, a newly isolated hantavirus from the Ussuri white-toothed shrew (Crocidura lasiura)
Abstract
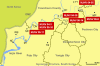
Until recently, the single known exception to the rodent-hantavirus association was Thottapalayam virus (TPMV), a long-unclassified virus isolated from the Asian house shrew (Suncus murinus). Robust gene amplification techniques have now uncovered several genetically distinct hantaviruses from shrews in widely separated geographic regions. Here, we report the characterization of a newly identified hantavirus, designated Imjin virus (MJNV), isolated from the lung tissues of Ussuri white-toothed shrews of the species Crocidura lasiura (order Soricomorpha, family Soricidae, subfamily Crocidurinae) captured near the demilitarized zone in the Republic of Korea during 2004 and 2005. Seasonal trapping revealed the highest prevalence of MJNV infection during the autumn, with evidence of infected shrews' clustering in distinct foci. Also, marked male predominance among anti-MJNV immunoglobulin G antibody-positive Ussuri shrews was found, whereas the male-to-female ratio among seronegative Ussuri shrews was near 1. Plaque reduction neutralization tests showed no cross neutralization for MJNV and rodent-borne hantaviruses but one-way cross neutralization for MJNV and TPMV. The nucleotide and deduced amino acid sequences for the different MJNV genomic segments revealed nearly the same calculated distances from hantaviruses harbored by rodents in the subfamilies Murinae, Arvicolinae, Neotominae, and Sigmodontinae. Phylogenetic analyses of full-length S, M, and L segment sequences demonstrated that MJNV shared a common ancestry with TPMV and remained in a distinct out-group, suggesting early evolutionary divergence. Studies are in progress to determine if MJNV is pathogenic for humans.
Figures




References
-
- Arai, S., S. N. Bennett, L. Sumibcay, J. A. Cook, J.-W. Song, A. Hope, C. Parmenter, V. R. Nerurkar, T. L. Yates, and R. Yanagihara. 2008. Phylogenetically distinct hantaviruses in the masked shrew (Sorex cinereus) and dusky shrew (Sorex monticolus) in the United States. Am. J. Trop. Med. Hyg. 78348-351. - PMC - PubMed
-
- Arikawa, J., A. L. Schmaljohn, J. M. Dalrymple, and C. S. Schmaljohn. 1989. Characterization of Hantaan virus envelope glycoprotein antigenic determinants defined by monoclonal antibodies. J. Gen. Virol. 70615-624. - PubMed
-
- Baek, L. J., H. Kariwa, K. Lokugamage, K. Yoshimatsu, J. Arikawa, I. Takashima, J. I. Kang, S. S. Moon, S. Y. Chung, E. J. Kim, H. J. Kang, K.-J. Song, T. A. Klein, R. Yanagihara, and J.-W. Song. 2006. Soochong virus: a genetically distinct hantavirus isolated from Apodemus peninsulae in Korea. J. Med. Virol. 78290-297. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

