Improved adenovirus type 5 vector-mediated transduction of resistant cells by piggybacking on coxsackie B-adenovirus receptor-pseudotyped baculovirus
- PMID: 19357170
- PMCID: PMC2687387
- DOI: 10.1128/JVI.00012-09
Improved adenovirus type 5 vector-mediated transduction of resistant cells by piggybacking on coxsackie B-adenovirus receptor-pseudotyped baculovirus
Abstract
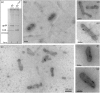
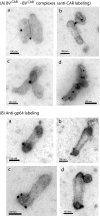
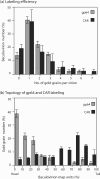
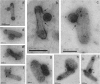
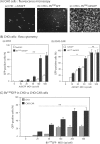
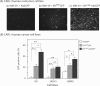
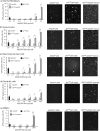
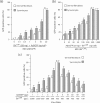
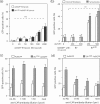
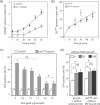
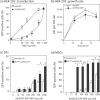
Taking advantage of the wide tropism of baculoviruses (BVs), we constructed a recombinant BV (BV(CAR)) pseudotyped with human coxsackie B-adenovirus receptor (CAR), the high-affinity attachment receptor for adenovirus type 5 (Ad5), and used the strategy of piggybacking Ad5-green fluorescent protein (Ad5GFP) vector on BV(CAR) to transduce various cells refractory to Ad5 infection. We found that transduction of all cells tested, including human primary cells and cancer cell lines, was significantly improved using the BV(CAR)-Ad5GFP biviral complex compared to that obtained with Ad5GFP or BV(CAR)GFP alone. We determined the optimal conditions for the formation of the complex and found that a high level of BV(CAR)-Ad5GFP-mediated transduction occurred at relatively low adenovirus vector doses, compared with transduction by Ad5GFP alone. The increase in transduction was dependent on the direct coupling of BV(CAR) to Ad5GFP via CAR-fiber knob interaction, and the cell attachment of the BV(CAR)-Ad5GFP complex was mediated by the baculoviral envelope glycoprotein gp64. Analysis of the virus-cell binding reaction indicated that the presence of BV(CAR) in the complex provided kinetic benefits to Ad5GFP compared to the effects with Ad5GFP alone. The endocytic pathway of BV(CAR)-Ad5GFP did not require Ad5 penton base RGD-integrin interaction. Biodistribution of BV(CAR)-Ad5Luc complex in vivo was studied by intravenous administration to nude BALB/c mice and compared to Ad5Luc injected alone. No significant difference in viscerotropism was found between the two inocula, and the liver remained the preferred localization. In vitro, coagulation factor X drastically increased the Ad5GFP-mediated transduction of CAR-negative cells but had no effect on the efficiency of transduction by the BV(CAR)-Ad5GFP complex. Various situations in vitro or ex vivo in which our BV(CAR)-Ad5 duo could be advantageously used as gene transfer biviral vector are discussed.
Figures

Similar articles
-
Efficient gene transfer into human CD34(+) cells by a retargeted adenovirus vector.J Virol. 2000 Mar;74(6):2567-83. doi: 10.1128/jvi.74.6.2567-2583.2000. J Virol. 2000. PMID: 10684271 Free PMC article.
-
Enhancement of adenovirus-mediated gene delivery to rheumatoid arthritis synoviocytes and synovium by fiber modifications: role of arginine-glycine-aspartic acid (RGD)- and non-RGD-binding integrins.J Immunol. 2005 Dec 1;175(11):7687-98. doi: 10.4049/jimmunol.175.11.7687. J Immunol. 2005. PMID: 16301679
-
Adenovirus serotype 30 fiber does not mediate transduction via the coxsackie-adenovirus receptor.J Virol. 2002 Jan;76(2):656-61. doi: 10.1128/jvi.76.2.656-661.2002. J Virol. 2002. PMID: 11752156 Free PMC article.
-
Tropism and transduction of oncolytic adenovirus 5 vectors in cancer therapy: Focus on fiber chimerism and mosaicism, hexon and pIX.Virus Res. 2018 Sep 15;257:40-51. doi: 10.1016/j.virusres.2018.08.012. Epub 2018 Aug 17. Virus Res. 2018. PMID: 30125593 Review.
-
Adenovirus vectors composed of subgroup B adenoviruses.Curr Gene Ther. 2007 Aug;7(4):229-38. doi: 10.2174/156652307781369137. Curr Gene Ther. 2007. PMID: 17969556 Review.
Cited by
-
PUMA gene delivery to synoviocytes reduces inflammation and degeneration of arthritic joints.Nat Commun. 2017 Jul 27;8(1):146. doi: 10.1038/s41467-017-00142-1. Nat Commun. 2017. PMID: 28747638 Free PMC article.
-
Cell entry and trafficking of human adenovirus bound to blood factor X is determined by the fiber serotype and not hexon:heparan sulfate interaction.PLoS One. 2011;6(5):e18205. doi: 10.1371/journal.pone.0018205. Epub 2011 May 26. PLoS One. 2011. PMID: 21637339 Free PMC article.
-
Baculovirus-assisted Reovirus Infection in Monolayer and Spheroid Cultures of Glioma cells.Sci Rep. 2017 Dec 15;7(1):17654. doi: 10.1038/s41598-017-17709-z. Sci Rep. 2017. PMID: 29247249 Free PMC article.
-
A novel platform for virus-like particle-display of flaviviral envelope domain III: induction of Dengue and West Nile virus neutralizing antibodies.Virol J. 2013 Apr 24;10:129. doi: 10.1186/1743-422X-10-129. Virol J. 2013. PMID: 23617954 Free PMC article.
-
Microparticle-mediated transfer of the viral receptors CAR and CD46, and the CFTR channel in a CHO cell model confers new functions to target cells.PLoS One. 2012;7(12):e52326. doi: 10.1371/journal.pone.0052326. Epub 2012 Dec 20. PLoS One. 2012. PMID: 23284987 Free PMC article.
References
-
- Ayres, M. D., S. C. Howard, J. Kuzio, M. Lopez-Ferber, and R. D. Possee. 1994. The complete DNA sequence of Autographa californica nuclear polyhedrosis virus. Virology 202586-605. - PubMed
-
- Bergelson, J. M., J. A. Cunningham, G. Droguett, E. A. Kurt-Jones, A. Krithivas, J. S. Hong, M. S. Horwitz, R. L. Crowell, and R. W. Finberg. 1997. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science 2751320-1323. - PubMed
-
- Boudin, M.-L., M. Moncany, J.-C. D'Halluin, and P. Boulanger. 1979. Isolation and characterization of adenovirus type 2 vertex capsomer (penton base). Virology 92125-138. - PubMed
-
- Boulanger, P., and F. Puvion. 1973. Large-scale preparation of soluble adenovirus hexon, penton and fiber antigens in highly purified form. Eur. J. Biochem. 3937-42. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

