RhCG is the major putative ammonia transporter expressed in the human kidney, and RhBG is not expressed at detectable levels
- PMID: 19357182
- PMCID: PMC2692438
- DOI: 10.1152/ajprenal.00013.2009
RhCG is the major putative ammonia transporter expressed in the human kidney, and RhBG is not expressed at detectable levels
Abstract
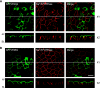
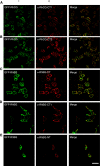
Rhesus glycoprotein homologs RhAG, RhBG, and RhCG comprise a recently identified branch of the Mep/Amt ammonia transporter family. Animal studies have shown that RhBG and RhCG are present in the kidney distal tubules. Studies in mouse and rat tissue suggest a basolateral localization for RhBG in cells of the distal tubules including the alpha-intercalated cells (alpha-IC), but no localization of RhBG has been reported in human tissue. To date RhCG localization has been described as exclusively apical plasma membrane in mouse and rat kidney, or apical and basolateral in humans, and some mouse and rat tissue studies. We raised novel antibodies to RhBG and RhCG to examine their localization in the human kidney. Madin-Darby canine kidney (MDCKI) cell lines stably expressing human green fluorescent protein-tagged RhBG or RhCG and human tissue lysates were used to demonstrate the specificity of these antibodies for detecting RhBG and RhCG. Using immunoperoxidase staining and antigen liberation techniques, both apical and basolateral RhCG localization was observed in the majority of the cells of the distal convoluted tubule and IC of the connecting tubule and collecting duct. Confocal microscopic imaging of normal human kidney cryosections showed that RhCG staining was predominantly localized to the apical membrane in these cells with some basolateral and intracellular staining evident. A proportion of RhCG staining labeled kAE1-positive cells, confirming that RhCG is localized to the alpha-IC cells. Surprisingly, no RhBG protein was detectable in the human kidney by Western blot analysis of tissue lysates, or by immunohistochemistry or confocal microscopy of tissue sections. The same antibodies, however, could detect RhBG in rat tissue. We conclude that under normal conditions, RhCG is the major putative ammonia transporter expressed in the human kidney and RhBG is not expressed at detectable levels.
Figures





References
-
- Bakouh N, Benjelloun F, Hulin P, Brouillard F, Edelman A, Cherif-Zahar B, Planelles G. NH3 is involved in the NH4+ transport induced by the functional expression of the human Rh C glycoprotein. J Biol Chem 279: 15975–15983, 2004. - PubMed
-
- Biner HL, Arpin-Bott MP, Loffing J, Wang X, Knepper M, Hebert SC, Kaissling B. Human cortical distal nephron: distribution of electrolyte and water transport pathways. J Am Soc Nephrol 13: 836–847, 2002. - PubMed
-
- Biver S, Belge H, Bourgeois S, Van Vooren P, Nowik M, Scohy S, Houillier P, Szpirer J, Szpirer C, Wagner CA, Devuyst O, Marini AM. A role for Rhesus factor Rhcg in renal ammonium excretion and male fertility. Nature 456: 339–343, 2008. - PubMed
-
- Bostanjoglo M, Reeves WB, Reilly RF, Velazquez H, Robertson N, Litwack G, Morsing P, Dorup J, Bachmann S, Ellison DH. 11Beta-hydroxysteroid dehydrogenase, mineralocorticoid receptor, and thiazide-sensitive Na-Cl cotransporter expression by distal tubules. J Am Soc Nephrol 9: 1347–1358, 1998. - PubMed
-
- Bruce LJ, Beckmann R, Ribeiro ML, Peters LL, Chasis JA, Delaunay J, Mohandas N, Anstee DJ, Tanner MJ. A band 3-based macrocomplex of integral and peripheral proteins in the RBC membrane. Blood 101: 4180–4188, 2003. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

