Agalsidase alfa and kidney dysfunction in Fabry disease
- PMID: 19357250
- PMCID: PMC2678048
- DOI: 10.1681/ASN.2008080870
Agalsidase alfa and kidney dysfunction in Fabry disease
Abstract
In male patients with Fabry disease, an X-linked disorder of glycosphingolipid metabolism caused by deficient activity of the lysosomal enzyme alpha-galactosidase A, kidney dysfunction becomes apparent by the third decade of life and invariably progresses to ESRD without treatment. Here, we summarize the effects of agalsidase alfa on kidney function from three prospective, randomized, placebo-controlled trials and their open-label extension studies involving 108 adult male patients. The mean baseline GFR among 54 nonhyperfiltrating patients (measured GFR <135 ml/min per 1.73 m(2)) treated with placebo was 85.4 +/- 29.6 ml/min per 1.73 m(2); during 6 mo of placebo, the mean annualized rate of change in GFR was -7.0 +/- 32.9 ml/min per 1.73 m(2). Among 85 nonhyperfiltrating patients treated with agalsidase alfa, the annualized rate of change was -2.9 +/- 8.7 ml/min per 1.73 m(2). Treatment with agalsidase alfa did not affect proteinuria. Multivariate analysis revealed that GFR and proteinuria category (< 1 or > or = 1 g/d) at baseline significantly predicted the rate of decline of GFR during treatment. This summary represents the largest group of male patients who had Fabry disease and for whom the effects of enzyme replacement therapy on kidney function have been studied. These data suggest that agalsidase alfa may stabilize kidney function in these patients.
Figures

 ), agalsidase alfa (□), or both placebo and agalsidase alfa. The number of patients is noted in each treatment box.
), agalsidase alfa (□), or both placebo and agalsidase alfa. The number of patients is noted in each treatment box.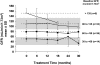



References
-
- Brady RO, Gal AE, Bradley RM, Martensson E, Warshaw AL, Laster L: Enzymatic defect in Fabry's disease: Ceramidetrihexosidase deficiency. N Engl J Med 276: 1163–1167, 1967 - PubMed
-
- Eng CM, Niehaus DJ, Enriquez AL, Burgert TS, Ludman MD, Desnick RJ: Fabry disease: Twenty-three mutations including sense and antisense CpG alterations and identification of a deletional hot-spot in the alpha-galactosidase A gene. Hum Mol Genet 3: 1795–1799, 1994 - PubMed
-
- Meikle PJ, Hopwood JJ, Clague AE, Carey WF: Prevalence of lysosomal storage disorders. JAMA 281: 249–254, 1999 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

