Neural stem and progenitor cells retain their potential for proliferation and differentiation into functional neurons despite lower number in aged brain
- PMID: 19357268
- PMCID: PMC6665731
- DOI: 10.1523/JNEUROSCI.6003-08.2009
Neural stem and progenitor cells retain their potential for proliferation and differentiation into functional neurons despite lower number in aged brain
Abstract
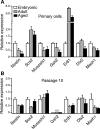
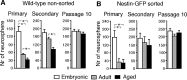
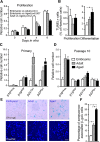
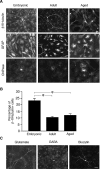
Neurogenesis in the subventricular zone (SVZ), which gives rise to new neurons in the olfactory bulb, continues throughout life but declines with increasing age. Little is known about how aging affects the intrinsic properties of the neural stem and progenitor cells (NSCs) in SVZ and the functional characteristics of their neuronal progeny. Here, we have compared the properties of NSCs isolated from embryonic lateral ganglionic eminence and adult and aged SVZ in mice using in vivo and in vitro systems, analyzed their gene expression profile, and studied their electrophysiological characteristics before and after differentiation into neurons. We show a loss of NSCs in SVZ from aged mice accompanied by reduced expression of genes for NSC markers, developmentally important transcription factors, and neurogenic factors. However, when isolated in vitro, the NSCs from SVZ of aged animals have capacity for proliferation and multilineage differentiation, including production of functional neurons, similar to that of NSCs in adult mice, albeit with lower efficacy. These properties are of major importance when considering therapeutic applications of neuronal replacement from endogenous NSCs in the injured, aged brain.
Figures




References
-
- Bailey KJ, Maslov AY, Pruitt SC. Accumulation of mutations and somatic selection in aging neural stem/progenitor cells. Aging Cell. 2004;3:391–397. - PubMed
-
- Bortner CD, Hughes FM, Jr, Cidlowski JA. A primary role for K+ and Na+ efflux in the activation of apoptosis. J Biol Chem. 1997;272:32436–32442. - PubMed
-
- Brown JP, Couillard-Després S, Cooper-Kuhn CM, Winkler J, Aigner L, Kuhn HG. Transient expression of doublecortin during adult neurogenesis. J Comp Neurol. 2003;467:1–10. - PubMed
-
- Cameron HA, McKay RD. Restoring production of hippocampal neurons in old age. Nat Neurosci. 1999;2:894–897. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
