Cooperative regulation of p53 by modulation of ternary complex formation with CBP/p300 and HDM2
- PMID: 19357310
- PMCID: PMC2672497
- DOI: 10.1073/pnas.0811023106
Cooperative regulation of p53 by modulation of ternary complex formation with CBP/p300 and HDM2
Abstract
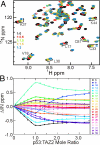
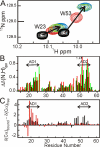
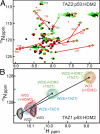
The tumor suppressor activity of p53 is regulated by interactions with the ubiquitin ligase HDM2 and the general transcriptional coactivators CBP and p300. Using NMR spectroscopy and isothermal titration calorimetry, we have dissected the binding interactions between the N-terminal transactivation domain (TAD) of p53, the TAZ1, TAZ2, KIX, and nuclear receptor coactivator binding domains of CBP, and the p53-binding domain of HDM2. The p53 TAD contains amphipathic binding motifs within the AD1 and AD2 regions that mediate interactions with CBP and HDM2. Binding of the p53 TAD to CBP domains is dominated by interactions with AD2, although the affinity is enhanced by additional interactions with AD1. In contrast, binding of p53 TAD to HDM2 is mediated primarily by AD1. The p53 TAD can bind simultaneously to HDM2 (through AD1) and to any one of the CBP domains (through AD2) to form a ternary complex. Phosphorylation of p53 at T18 impairs binding to HDM2 and enhances affinity for the CBP KIX domain. Multisite phosphorylation of the p53 TAD at S15, T18, and S20 leads to increased affinity for the TAZ1 and KIX domains of CBP. These observations suggest a mechanism whereby HDM2 and CBP/p300 function synergistically to regulate the p53 response. In unstressed cells, CBP/p300, HDM2 and p53 form a ternary complex that promotes polyubiquitination and degradation of p53. After cellular stress and DNA damage, p53 becomes phosphorylated at T18 and other residues in the AD1 region, releases HDM2 and binds preferentially to CBP/p300, leading to stabilization and activation of p53.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Mapping the interactions of the p53 transactivation domain with the KIX domain of CBP.Biochemistry. 2009 Mar 17;48(10):2115-24. doi: 10.1021/bi802055v. Biochemistry. 2009. PMID: 19220000 Free PMC article.
-
Structural insights into TAZ2 domain-mediated CBP/p300 recruitment by transactivation domain 1 of the lymphopoietic transcription factor E2A.J Biol Chem. 2020 Mar 27;295(13):4303-4315. doi: 10.1074/jbc.RA119.011078. Epub 2020 Feb 25. J Biol Chem. 2020. PMID: 32098872 Free PMC article.
-
Recognition of the disordered p53 transactivation domain by the transcriptional adapter zinc finger domains of CREB-binding protein.Proc Natl Acad Sci U S A. 2016 Mar 29;113(13):E1853-62. doi: 10.1073/pnas.1602487113. Epub 2016 Mar 14. Proc Natl Acad Sci U S A. 2016. PMID: 26976603 Free PMC article.
-
p300/CBP/p53 interaction and regulation of the p53 response.Eur J Biochem. 2001 May;268(10):2773-8. doi: 10.1046/j.1432-1327.2001.02226.x. Eur J Biochem. 2001. PMID: 11358491 Review.
-
Role of Intrinsic Protein Disorder in the Function and Interactions of the Transcriptional Coactivators CREB-binding Protein (CBP) and p300.J Biol Chem. 2016 Mar 25;291(13):6714-22. doi: 10.1074/jbc.R115.692020. Epub 2016 Feb 5. J Biol Chem. 2016. PMID: 26851278 Free PMC article. Review.
Cited by
-
p53 Acetylation: Regulation and Consequences.Cancers (Basel). 2014 Dec 23;7(1):30-69. doi: 10.3390/cancers7010030. Cancers (Basel). 2014. PMID: 25545885 Free PMC article. Review.
-
Real-time and simultaneous monitoring of the phosphorylation and enhanced interaction of p53 and XPC acidic domains with the TFIIH p62 subunit.Oncogenesis. 2015 Jun 1;4(6):e150. doi: 10.1038/oncsis.2015.13. Oncogenesis. 2015. PMID: 26029824 Free PMC article.
-
Functional advantages of dynamic protein disorder.FEBS Lett. 2015 Sep 14;589(19 Pt A):2433-40. doi: 10.1016/j.febslet.2015.06.003. Epub 2015 Jun 11. FEBS Lett. 2015. PMID: 26073260 Free PMC article. Review.
-
The HTLV-1-encoded protein HBZ directly inhibits the acetyl transferase activity of p300/CBP.Nucleic Acids Res. 2012 Jul;40(13):5910-25. doi: 10.1093/nar/gks244. Epub 2012 Mar 19. Nucleic Acids Res. 2012. PMID: 22434882 Free PMC article.
-
ERK2 MAP kinase regulates SUFU binding by multisite phosphorylation of GLI1.Life Sci Alliance. 2022 Jul 13;5(11):e202101353. doi: 10.26508/lsa.202101353. Print 2022 Nov. Life Sci Alliance. 2022. PMID: 35831023 Free PMC article.
References
-
- Espinosa JM, Verdun RE, Emerson BM. p53 Functions through stress- and promoter-specific recruitment of transcription initiation components before and after DNA damage. Mol Cell. 2003;12:1015–1027. - PubMed
-
- Minsky N, Oren M. The RING domain of Mdm2 mediates histone ubiquitylation and transcriptional repression. Mol Cell. 2004;16:631–639. - PubMed
-
- Appella E, Anderson CW. Post-translational modifications and activation of p53 by genotoxic stresses. Eur J Biochem. 2001;268:2764–2772. - PubMed
-
- Bode AM, Dong Z. Post-translational modification of p53 in tumorigenesis. Nat Rev Cancer. 2004;4:793–805. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

