An avian basal ganglia-forebrain circuit contributes differentially to syllable versus sequence variability of adult Bengalese finch song
- PMID: 19357331
- PMCID: PMC2694116
- DOI: 10.1152/jn.91089.2008
An avian basal ganglia-forebrain circuit contributes differentially to syllable versus sequence variability of adult Bengalese finch song
Abstract
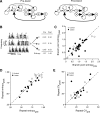
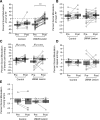
Behavioral variability is important for motor skill learning but continues to be present and actively regulated even in well-learned behaviors. In adult songbirds, two types of song variability can persist and are modulated by social context: variability in syllable structure and variability in syllable sequencing. The degree to which the control of both types of adult variability is shared or distinct remains unknown. The output of a basal ganglia-forebrain circuit, LMAN (the lateral magnocellular nucleus of the anterior nidopallium), has been implicated in song variability. For example, in adult zebra finches, neurons in LMAN actively control the variability of syllable structure. It is unclear, however, whether LMAN contributes to variability in adult syllable sequencing because sequence variability in adult zebra finch song is minimal. In contrast, Bengalese finches retain variability in both syllable structure and syllable sequencing into adulthood. We analyzed the effects of LMAN lesions on the variability of syllable structure and sequencing and on the social modulation of these forms of variability in adult Bengalese finches. We found that lesions of LMAN significantly reduced the variability of syllable structure but not of syllable sequencing. We also found that LMAN lesions eliminated the social modulation of the variability of syllable structure but did not detect significant effects on the modulation of sequence variability. These results show that LMAN contributes differentially to syllable versus sequence variability of adult song and suggest that these forms of variability are regulated by distinct neural pathways.
Figures




References
-
- Aronov D, Andalman AS, Fee MS. A specialized forebrain circuit for vocal babbling in the juvenile songbird. Science 320: 630–634, 2008. - PubMed
-
- Ball GF, Castelino CB, Maney DL, Appeltants D, Balthazart J. The activation of birdsong by testosterone: multiple sites of action and role of ascending catecholamine projections. Ann NY Acad Sci 1007: 211–231, 2003. - PubMed
-
- Bottjer SW Developmental regulation of basal ganglia circuitry during the sensitive period for vocal learning in songbirds. Ann NY Acad Sci 1016: 395–415, 2004. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

