Stress-induced changes in nucleus accumbens glutamate synaptic plasticity
- PMID: 19357347
- PMCID: PMC3817271
- DOI: 10.1152/jn.91111.2008
Stress-induced changes in nucleus accumbens glutamate synaptic plasticity
Abstract
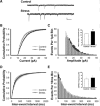
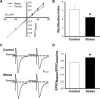
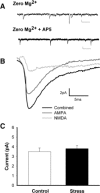
Stress hormones released in the CNS following exposure to unavoidable, aversive stimuli have been shown to alter the physiology of neurons in multiple brain regions including hippocampus, amygdala, prefrontal cortex, and ventral tegmental area. The nucleus accumbens (NAc), a motor-limbic interface linked to motivation and reward, receives inputs from each of these stress-affected brain regions, raising the possibility that its function might also be altered in response to stress. To assess potential stress-induced plasticity in the NAc, we exposed adult mice to daily cold water forced swim for 2 consecutive days and conducted electrophysiological experiments assessing glutamate receptor function in brain slices taken 18-24 h following the second swim. We found that AMPA receptor (AMPAR)/N-methyl-d-aspartate receptor (NMDAR) ratios, a measure of synaptic strength, were increased in the NAc shell but not core medium spiny neurons (MSNs) in stressed animals relative to controls. This effect was blocked by preadministration of glucocorticoid receptor (GR) antagonist RU486, suggesting that the observed changes are dependent on corticosteroid signaling. The role of corticosterone (CORT) in the observed plasticity was confirmed, because exogenous administration of 10 mg/kg CORT also enhanced AMPAR/NMDAR ratios in the NAc shell. The synaptic changes in NAc shell MSNs reflect an enhancement of AMPAR-mediated currents, as we observed increased AMPAR miniature postsynaptic current (mEPSC) amplitude following stress but no change in NMDAR mEPSCs. We hypothesize that altered information processing via plasticity of excitatory inputs might contribute to reward-related behaviors such as stress-induced reinstatement of drug seeking in animals and relapse in humans.
Figures


References
-
- Barrot M, Marinelli M, Abrous DN, Rouge-Pont F, Le Moal M, Piazza PV. Functional heterogeneity in dopamine release and in the expression of Fos-like proteins within the rat striatal complex. Eur J Neurosci 11: 1155–1166, 1999 - PubMed
-
- Barrot M, Marinelli M, Abrous DN, Rouge-Pont F, Le Moal M, Piazza PV. The dopaminergic hyper-responsiveness of the shell of the nucleus accumbens is hormone-dependent. Eur J Neurosci 12: 973–979, 2000 - PubMed
-
- Boudreau AC, Reimers JM, Milovanovic M, Wolf ME. Cell surface AMPA receptors in the rat nucleus accumbens increase during cocaine withdrawal but internalize after cocaine challenge in association with altered activation of mitogen-activated protein kinases. J Neurosci 27: 10621–10635, 2007 - PMC - PubMed
-
- Burnashev N, Monyer H, Seeburg PH, Sakmann B. Divalent ion permeability of AMPA receptor channels is dominated by the edited form of a single subunit. Neuron 8: 189–198, 1992 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

