CD4 and CD8 T cell responses to the M. tuberculosis Ag85B-TB10.4 promoted by adjuvanted subunit, adenovector or heterologous prime boost vaccination
- PMID: 19357780
- PMCID: PMC2663846
- DOI: 10.1371/journal.pone.0005139
CD4 and CD8 T cell responses to the M. tuberculosis Ag85B-TB10.4 promoted by adjuvanted subunit, adenovector or heterologous prime boost vaccination
Abstract
Background: Although CD4 T cells are crucial for defense against M.tb, it is still not clear whether the optimal response against M.tb in fact involves both CD4 and CD8 T cells. To test this, we used a new vaccine strategy that generated a strong balanced T cell response consisting of both CD4 and CD8 T cells.
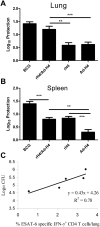
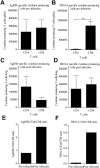
Methods and findings: To compare CD4 and CD8 responses against Ag85B-TB10.4 (H4), H4 was delivered as a subunit vaccine in cationic liposomes (CAF01), expressed in Ad5 (Ad-H4) or as a heterologous prime boost vaccination. H4/CAF01 induced primarily CD4 T cells and Ad-H4 gave predominantly a CD8 T cell response. In contrast, the heterologous prime boost combination resulted in augmentation of both the CD4 and CD8 response. The majority (>40%) of the CD4 T cells induced by the heterologous prime boost protocol were polyfunctional, and expressed IFN-gamma(+), IL-2(+), and TNF-alpha(+), whereas most of the CD8 T cells expressed IFN-gamma(+) and TNF-alpha(+) and possessed strong cytotoxic potential. The heterologous prime boost protocol also gave an increase in protective efficacy against M.tb challenge compared to H4/CAF01 and Ad-H4. Both the H4 specific CD4 and CD8 T cells were recruited to the site of infection, at the onset of infection. However, compared to CD8 T cells, CD4 T cells showed more extensive recruitment and were the main T cell subset proliferating at the site of infection.
Conclusions/significance: Heterologous prime boost based on H4, produced an additive effect on the priming of CD4 and CD8 cells and in terms of the protective capacity of the vaccine, and therefore represent an interesting new vaccine strategy against M.tb. However, CD4 and CD8 T cells respond very differently to live M.tb challenge, in a manner which supports the consensus that CD4 T cells do play the major role during the early stages of an M.tb infection.
Conflict of interest statement
Figures





References
-
- Dorman SE, Holland SM. Interferon-gamma and interleukin-12 pathway defects and human disease. Cytokine Growth Factor Rev. 2000;11:321–333. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

