Induction of osteopontin expression by nicotine and cigarette smoke in the pancreas and pancreatic ductal adenocarcinoma cells
- PMID: 19358273
- PMCID: PMC4465299
- DOI: 10.1002/ijc.24388
Induction of osteopontin expression by nicotine and cigarette smoke in the pancreas and pancreatic ductal adenocarcinoma cells
Abstract
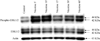
Pancreatic ductal adenocarcinoma (PDA) is a lethal disease with etiological association with cigarette smoking. Nicotine, an important component of cigarettes, exists at high concentrations in the bloodstream of smokers. Osteopontin (OPN) is a secreted phosphoprotein that confers on cancer cells a migratory phenotype and activates signaling pathways that induce cell survival, proliferation, invasion, and metastasis. Here, we investigated the potential molecular basis of nicotine's role in PDA through studying its effect on OPN. Nicotine significantly (p < 0.02) increased OPN mRNA and protein secretion in PDA cells through activation of the OPN gene promoter. The OPN mRNA induction was inhibited by the nicotinic acetylcholine receptor antagonist, mechamylamine. Further, the tyrosine kinase inhibitor genistein inhibited the nicotine-mediated induction of OPN, suggesting that mitogen activated protein kinase signaling mechanism is involved. Nicotine activated the phosphorylation of ERK1/2, but not p38 or c-Jun NH2-terminal MAP kinases. Inhibition of ERK1/2 activation reduced the nicotine-induced OPN synthesis. Rats exposed to cigarette smoke showed a dose-dependent increase in pancreatic OPN that paralleled the rise of pancreatic and plasma nicotine levels. Analysis of cancer tissue from invasive PDA patients, the majority of whom were smokers, showed the presence of significant amounts of OPN in the malignant ducts and the surrounding pancreatic acini. Our data suggest that nicotine may contribute to PDA pathogenesis through upregulation of OPN. They provide the first insight into a nicotine-initiated signal transduction pathway that regulates OPN as a possible tumorigenic mechanism in PDA.
Copyright 2009 UICC.
Figures






















Similar articles
-
Expression and regulation of nicotine receptor and osteopontin isoforms in human pancreatic ductal adenocarcinoma.Histol Histopathol. 2011 Jul;26(7):893-904. doi: 10.14670/HH-26.893. Histol Histopathol. 2011. PMID: 21630219
-
Induction of monocyte chemoattractant protein-1 by nicotine in pancreatic ductal adenocarcinoma cells: role of osteopontin.Surgery. 2010 Aug;148(2):298-309. doi: 10.1016/j.surg.2010.05.002. Epub 2010 Jun 25. Surgery. 2010. PMID: 20579680 Free PMC article.
-
Involvement of osteopontin in the matrix-degrading and proangiogenic changes mediated by nicotine in pancreatic cancer cells.J Gastrointest Surg. 2010 Oct;14(10):1566-77. doi: 10.1007/s11605-010-1338-0. Epub 2010 Sep 8. J Gastrointest Surg. 2010. PMID: 20824368
-
Expression of a prometastatic splice variant of osteopontin, OPNC, in human pancreatic ductal adenocarcinoma.Surgery. 2009 Aug;146(2):232-40. doi: 10.1016/j.surg.2009.03.036. Surgery. 2009. PMID: 19628079 Free PMC article.
-
Angiotensin II induces vascular endothelial growth factor in pancreatic cancer cells through an angiotensin II type 1 receptor and ERK1/2 signaling.J Gastrointest Surg. 2008 Jan;12(1):57-66. doi: 10.1007/s11605-007-0403-9. Epub 2007 Nov 17. J Gastrointest Surg. 2008. PMID: 18026817
Cited by
-
Beta-adrenergic signaling in the development and progression of pulmonary and pancreatic adenocarcinoma.Curr Cancer Ther Rev. 2012 May 1;8(2):116-127. doi: 10.2174/157339412800675351. Curr Cancer Ther Rev. 2012. PMID: 23807873 Free PMC article.
-
Acetylcholine Sustains LNCaP Prostate Cancer Cell Migration, Invasion and Proliferation Through Glyoxalase 1/MG-H1 Axis with the Involvement of Osteopontin.Int J Mol Sci. 2025 Apr 25;26(9):4107. doi: 10.3390/ijms26094107. Int J Mol Sci. 2025. PMID: 40362346 Free PMC article.
-
Pancreatic cancer cells and normal pancreatic duct epithelial cells express an autocrine catecholamine loop that is activated by nicotinic acetylcholine receptors α3, α5, and α7.Mol Cancer Res. 2012 Feb;10(2):239-49. doi: 10.1158/1541-7786.MCR-11-0332. Epub 2011 Dec 21. Mol Cancer Res. 2012. PMID: 22188668 Free PMC article.
-
Nicotinic acetylcholine receptors and cancer.Biomed Rep. 2016 May;4(5):515-518. doi: 10.3892/br.2016.625. Epub 2016 Mar 7. Biomed Rep. 2016. PMID: 27123240 Free PMC article.
-
Nicotine induces pro-inflammatory response in aortic vascular smooth muscle cells through a NFκB/osteopontin amplification loop-dependent pathway.Inflammation. 2012 Feb;35(1):342-9. doi: 10.1007/s10753-011-9324-6. Inflammation. 2012. PMID: 21494800
References
-
- Jemal A, Siegel R, Ward E, Murray T, Thun MJ. Cancer Statistics, 2007. CA Cancer J Clin. 2007;57:43–66. - PubMed
-
- Khan SA, Cook AC, Kappil M, Günthert U, Chambers AF, Tuck AB, Denhardt DT. Enhanced cell surface CD44 variant (v6, v9) expression by osteopontin in breast cancer epithelial cells facilitates tumor cell migration: novel post-transcriptional, post-translational regulation. Clin Exp Metastasis. 2005;22(8):663–673. - PubMed
-
- Jain S, Chakraborty G, Bulbule A, Kaur R, Kundu GC. Osteopontin: an emerging therapeutic target for anticancer therapy. Expert Opin Ther Targets. 2007;1:81–90. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

