Development and plasticity of intra- and intersensory information processing
- PMID: 19358458
- PMCID: PMC3639492
- DOI: 10.3766/jaaa.19.10.6
Development and plasticity of intra- and intersensory information processing
Abstract
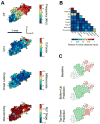
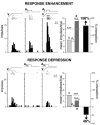
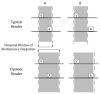
The functional architecture of sensory brain regions reflects an ingenious biological solution to the competing demands of a continually changing sensory environment. While they are malleable, they have the constancy necessary to support a stable sensory percept. How does the functional organization of sensory brain regions contend with these antithetical demands? Here we describe the functional organization of auditory and multisensory (i.e., auditory-visual) information processing in three sensory brain structures: (1) a low-level unisensory cortical region, the primary auditory cortex (A1); (2) a higher-order multisensory cortical region, the anterior ectosylvian sulcus (AES); and (3) a multisensory subcortical structure, the superior colliculus (SC). We then present a body of work that characterizes the ontogenic expression of experience-dependent influences on the operations performed by the functional circuits contained within these regions. We will present data to support the hypothesis that the competing demands for plasticity and stability are addressed through a developmental transition in operational properties of functional circuits from an initially labile mode in the early stages of postnatal development to a more stable mode in the mature brain that retains the capacity for plasticity under specific experiential conditions. Finally, we discuss parallels between the central tenets of functional organization and plasticity of sensory brain structures drawn from animal studies and a growing literature on human brain plasticity and the potential applicability of these principles to the audiology clinic.
Figures



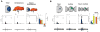

References
-
- Bakin JS, South DA, Weinberger NM. Induction of receptive field plasticity in the auditory cortex of the guinea pig during instrumental avoidance conditioning. Behav Neurosci. 1996;110(5):905–913. - PubMed
-
- Bakin JS, Weinberger NM. Classical conditioning induces CS-specific receptive field plasticity in the auditory cortex of the guinea pig. Brain Res. 1990;536(1-2):271–286. - PubMed
-
- Bao S, Chan VT, Merzenich MM. Cortical remodelling induced by activity of ventral tegmental dopamine neurons. Nature. 2001;412(6842):79–83. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

