Mechanism of the very efficient quenching of tryptophan fluorescence in human gamma D- and gamma S-crystallins: the gamma-crystallin fold may have evolved to protect tryptophan residues from ultraviolet photodamage
- PMID: 19358562
- PMCID: PMC2674318
- DOI: 10.1021/bi802177g
Mechanism of the very efficient quenching of tryptophan fluorescence in human gamma D- and gamma S-crystallins: the gamma-crystallin fold may have evolved to protect tryptophan residues from ultraviolet photodamage
Abstract
Proteins exposed to UV radiation are subject to irreversible photodamage through covalent modification of tryptophans (Trps) and other UV-absorbing amino acids. Crystallins, the major protein components of the vertebrate eye lens that maintain lens transparency, are exposed to ambient UV radiation throughout life. The duplicated beta-sheet Greek key domains of beta- and gamma-crystallins in humans and all other vertebrates each have two conserved buried Trps. Experiments and computation showed that the fluorescence of these Trps in human gammaD-crystallin is very efficiently quenched in the native state by electrostatically enabled electron transfer to a backbone amide [Chen et al. (2006) Biochemistry 45, 11552-11563]. This dispersal of the excited state energy would be expected to minimize protein damage from covalent scission of the excited Trp ring. We report here both experiments and computation showing that the same fast electron transfer mechanism is operating in a different crystallin, human gammaS-crystallin. Examination of solved structures of other crystallins reveals that the Trp conformation, as well as favorably oriented bound waters, and the proximity of the backbone carbonyl oxygen of the n - 3 residues before the quenched Trps (residue n), are conserved in most crystallins. These results indicate that fast charge transfer quenching is an evolved property of this protein fold, probably protecting it from UV-induced photodamage. This UV resistance may have contributed to the selection of the Greek key fold as the major lens protein in all vertebrates.
Figures
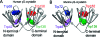


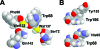
References
-
- Jun S. H.; Kim T. G.; Ban C. (2006) DNA mismatch repair system. Classical and fresh roles. FEBS J. 273, 1609–1619. - PubMed
-
- White M. F. (2003) Archaeal DNA repair: paradigms and puzzles. Biochem. Soc. Trans. 31, 690–693. - PubMed
-
- Davies M. J.; Truscott R. J. (2001) Photo-oxidation of proteins and its role in cataractogenesis. J. Photochem. Photobiol. B 63, 114–125. - PubMed
-
- Grossweiner L. I. (1984) Photochemistry of proteins: a review. Curr. Eye Res. 3, 137–144. - PubMed
-
- Barber J.; Andersson B. (1992) Too much of a good thing: light can be bad for photosynthesis. Trends Biochem. Sci. 17, 61–66. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

