Mechanisms and function of DUOX in epithelia of the lung
- PMID: 19358684
- PMCID: PMC2823369
- DOI: 10.1089/ars.2009.2558
Mechanisms and function of DUOX in epithelia of the lung
Abstract
The human lung produces considerable amounts of H(2)O(2). In the normal uninflamed epithelium of both the airways and the alveoli, mucosal release of H(2)O(2) is readily detected both in cell cultures in vitro and in the exhaled breath of humans. The dual oxidases DUOX1 and DUOX2 are the H(2)O(2)-producing isoforms of the NADPH oxidase family found in epithelial cells. The DUOXs are prominently expressed at the apical cell pole of ciliated cells in the airways and in type II cells of the alveoli. Recent studies focused on the functional consequences of H(2)O(2) release by DUOX into the lung lining fluid. In the airways, a major function of DUOX is to support lactoperoxidase (LPO) to generate bactericidal OSCN(-), and there are indications that the DUOX/LPO defense system is critically dependent on the function of the CFTR Cl(-) channel, which provides both SCN(-) (for LPO function) and HCO(3)(-) (for pH adjustment) to the airway surface liquid. Although DUOX is also functional in the alveolar epithelium, no comparable heme peroxidase is present in the alveolus, and thus DUOX-mediated H(2)O(2) release by alveolar cells may have other functions, such as cellular signaling.
Figures
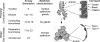


References
-
- Al–Obaidy A. Al–Samarai A. Exhaled breath condensate pH and hydrogen peroxide as non-invasive markers for asthma. Saudi Med J. 2007;28:1860–1863. - PubMed
-
- Ameziane–El-Hassani R. Morand S. Boucher J–L. Frapart Y–M. Apostolou D. Agnandji D. Gnidehou S. Ohayon R. Noel–Hudson M–S. Francon J. Lalaoui K. Virion A. Dupuy C. Dual oxidase-2 has an intrinsic Ca2+-dependent H2O2-generating activity. J Biol Chem. 2005;2280:30046–30054. - PubMed
-
- Antczak A. Kurmanowska Z. Kasielski M. Nowak D. Inhaled glucocorticosteroids decrease hydrogen peroxide level in expired air condensate in asthmatic patients. Respir Med. 2000;94:416–421. - PubMed
-
- Antczak A. Nowak D. Shariati B. Krol M. Piasecka G. Kurmanowska Z. Increased hydrogen peroxide and thiobarbituric acid-reactive products in expired breath condensate of asthmatic patients. Eur Respir J. 1997;10:1235–1241. - PubMed
-
- Ashino Y. Ying X. Dobbs LG. Bhattacharya J. [Ca2+]i oscillations regulate type II cell exocytosis in the pulmonary alveolus. Am J Physiol Lung Cell Mol Physiol. 2000;279:L5–13. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

