A randomized, controlled trial of initial anti-retroviral therapy with abacavir/lamivudine/zidovudine twice-daily compared to atazanavir once-daily with lamivudine/zidovudine twice-daily in HIV-infected patients over 48 weeks (ESS100327, the ACTION Study)
- PMID: 19358725
- PMCID: PMC2672933
- DOI: 10.1186/1742-6405-6-3
A randomized, controlled trial of initial anti-retroviral therapy with abacavir/lamivudine/zidovudine twice-daily compared to atazanavir once-daily with lamivudine/zidovudine twice-daily in HIV-infected patients over 48 weeks (ESS100327, the ACTION Study)
Abstract
Background: Traditional first line regimens containing a non-nucleoside reverse transcriptase inhibitor or protease inhibitor may not be suitable for a subset of antiretroviral-naïve patients such as those with certain co-morbidities, women of child-bearing potential, and intolerability to components of standard first line therapy. This study was conducted to determine if alternate treatment options may meet the needs of both general and special patient populations. The ACTION study was a randomized, open-label, multicenter, 48-week trial that compared the safety and efficacy of a triple nucleoside regimen versus a protease inhibitor plus a dual nucleoside regimen in HIV-1 treatment-naïve subjects.
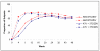
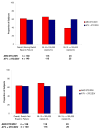
Results: 279 HIV-infected subjects with HIV-1 RNA (VL) >5000 but < 200,000 copies/mL (c/mL) and CD4+ count >/= 100 cells/mm3 were randomized (1:1) to receive abacavir sulfate/lamivudine/zidovudine (ABC/3TC/ZDV) twice-daily or atazanavir (ATV) once-daily plus lamivudine/zidovudine (3TC/ZDV) twice-daily. Protocol-defined virologic failure was based on multiple failure criteria.Non-inferiority of ABC/3TC/ZDV to ATV+3TC/ZDV was established with 62% vs. 59% of subjects achieving a VL < 50 c/mL at week 48, [ITT(E), M/S = F, 95% CI: -5.9, 10.4]. Similar results were observed in the 230 (82%) subjects with baseline VL<100,000 c/mL (ABC/3TC/ZDV vs. ATV+3TC/ZDV), 66% vs. 59%; 95% CI: -5.6, 19.5. However, ABC/3TC/ZDV did not meet the non-inferiority criterion compared to ATV+3TC/ZDV in the 48 subjects with baseline VL >/= 100,000 c/mL, 39% vs. 60%; 95% CI: -49.2, 7.4, respectively. Protocol-defined virologic failure was similar between groups.
Conclusion: ABC/3TC/ZDV demonstrated comparable virologic efficacy to ATV+3TC/ZDV in this population over 48 weeks. In those with a baseline VL >/= 100,000 c/mL, subjects in the ATV+3TC/ZDV showed better virologic efficacy. Both regimens offer benefits in select therapy-naïve subjects.
Trial registration: [Clinical Trials Identifier, NCT00082394].
Figures

Similar articles
-
A randomized, double-blind study of triple nucleoside therapy of abacavir, lamivudine, and zidovudine versus lamivudine and zidovudine in previously treated human immunodeficiency virus type 1-infected children. The CNAA3006 Study Team.Pediatrics. 2001 Jan;107(1):E4. doi: 10.1542/peds.107.1.e4. Pediatrics. 2001. PMID: 11134468 Clinical Trial.
-
A randomized controlled trial of single-class maintenance therapy with abacavir/lamivudine/zidovudine after standard triple antiretroviral induction therapy: final 96-week results from the FREE study.HIV Med. 2015 Feb;16(2):122-31. doi: 10.1111/hiv.12186. Epub 2014 Dec 4. HIV Med. 2015. PMID: 25472825 Clinical Trial.
-
Induction with abacavir/lamivudine/zidovudine plus efavirenz for 48 weeks followed by 48-week maintenance with abacavir/lamivudine/zidovudine alone in antiretroviral-naive HIV-1-infected patients.J Acquir Immune Defic Syndr. 2005 Jul 1;39(3):257-64. doi: 10.1097/01.qai.0000169664.15536.20. J Acquir Immune Defic Syndr. 2005. PMID: 15980684 Clinical Trial.
-
Effects of nucleoside reverse transcriptase inhibitor backbone on the efficacy of first-line boosted highly active antiretroviral therapy based on protease inhibitors: meta-regression analysis of 12 clinical trials in 5168 patients.HIV Med. 2009 Oct;10(9):527-35. doi: 10.1111/j.1468-1293.2009.00724.x. HIV Med. 2009. PMID: 19785663 Review.
-
Triple-nucleoside analog antiretroviral therapy: is there still a role in clinical practice? A review.MedGenMed. 2005 Jun 2;7(2):70. MedGenMed. 2005. PMID: 16369448 Free PMC article. Review.
Cited by
-
Antiretroviral regimens in pregnancy and breast-feeding in Botswana.N Engl J Med. 2010 Jun 17;362(24):2282-94. doi: 10.1056/NEJMoa0907736. N Engl J Med. 2010. PMID: 20554983 Free PMC article. Clinical Trial.
-
Poor early virologic performance and durability of abacavir-based first-line regimens for HIV-infected children.Pediatr Infect Dis J. 2013 Aug;32(8):851-5. doi: 10.1097/INF.0b013e31828c3738. Pediatr Infect Dis J. 2013. PMID: 23860481 Free PMC article.
-
Abacavir-based triple nucleoside regimens for maintenance therapy in patients with HIV.Cochrane Database Syst Rev. 2013 Jun 5;2013(6):CD008270. doi: 10.1002/14651858.CD008270.pub2. Cochrane Database Syst Rev. 2013. PMID: 23740608 Free PMC article.
-
When to start, what to start and other treatment controversies in pediatric HIV infection.Paediatr Drugs. 2012 Dec 1;14(6):361-76. doi: 10.2165/11599640-000000000-00000. Paediatr Drugs. 2012. PMID: 23013459
-
Changes from 2000 to 2009 in the Prevalence of HIV-1 Containing Drug Resistance-Associated Mutations from Antiretroviral Therapy-Naive, HIV-1-Infected Patients in the United States.AIDS Res Hum Retroviruses. 2018 Aug;34(8):672-679. doi: 10.1089/AID.2017.0295. Epub 2018 Jun 5. AIDS Res Hum Retroviruses. 2018. PMID: 29732898 Free PMC article.
References
-
- Panel on Clinical Practices for the Treatment of HIV Infection Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services and Henry J. Kaiser Foundation http://aidsinfo.nih.gov/guidelines
-
- Staszewski S, Keiser P, Montaner J, Raffi F, Gathe J, Brotas V, Hicks C, Hammer SM, Cooper D, Johnson M, Tortell S, Cutrell A, Thorborn D, Isaacs R, Hetherington S, Steel H, Spreen W. CNAAB3005 International Study Team. Abacavir-lamivudine-zidovudine vs indinavir-lamivudine-zidovudine in antiretroviral-naive HIV-infected adults: A randomized equivalence trial. JAMA. 2001;285:1155–63. doi: 10.1001/jama.285.9.1155. - DOI - PubMed
-
- Vibhagool A, Capn P, Schechter M, Smaill F, Soto-Ramirez L, Carosi G, Montroni M, Pharo CE, Jordan JC, Thomas NE, Pearce G. Triple nucleoside treatment with abacavir plus the lamivudine/zidovudine combination tablet (COM) compared to indinavir/COM in antiretroviral therapy-naive adults: results of a 48-week open-label, equivalence trial (CNA3014) Curr Med Res Opin. 2004;20:1103–14. doi: 10.1185/030079904125004006. - DOI - PubMed
-
- Kumar PN, Rodriguez-French A, Thompson MA, Tashima KT, Averitt D, Wannamaker PG, Williams VC, Shaefer MS, Pakes GE, Pappa KA, ESS40002 Study Team A prospective, 96-week study of the impact of Trizivir, Combivir/nelfinavir, and lamivudine/stavudine/nelfinavir on lipids, metabolic parameters and efficacy in antiretroviral-naive patients: effect of sex and ethnicity. HIV Med. 2006;7:85–98. doi: 10.1111/j.1468-1293.2006.00346.x. - DOI - PubMed
-
- Gulick RM, Ribaudo HJ, Shikuma CM, Lustgarten S, Squires KE, Meyer WA, 3rd, Acosta EP, Schackman BR, Pilcher CD, Murphy RL, Maher WE, Witt MD, Reichman RC, Snyder S, Klingman KL, Kuritzkes DR, AIDS Clinical Trials Group Study A5095 Team Triple-nucleoside regimens versus efavirenz-containing regimens for the initial treatment of HIV-1 infection. N Engl J Med. 2004;350:1850–61. doi: 10.1056/NEJMoa031772. - DOI - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

