Discovery and exploitation of inhibitor-resistant aurora and polo kinase mutants for the analysis of mitotic networks
- PMID: 19359241
- PMCID: PMC2708884
- DOI: 10.1074/jbc.M109.005694
Discovery and exploitation of inhibitor-resistant aurora and polo kinase mutants for the analysis of mitotic networks
Abstract
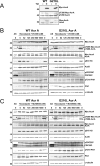
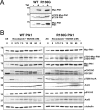
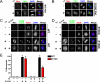
The Aurora and Polo-like kinases are central components of mitotic signaling pathways, and recent evidence suggests that substantial cross-talk exists between Aurora A and Plk1. In addition to their validation as novel anticancer agents, small molecule kinase inhibitors are increasingly important tools to help dissect clinically relevant protein phosphorylation networks. However, one major problem associated with kinase inhibitors is their promiscuity toward "off-target" members of the kinome, which makes interpretation of data obtained from complex cellular systems challenging. Additionally, the emergence of inhibitor resistance in patients makes it clear that an understanding of resistance mechanisms is essential to inform drug design. In this study, we exploited structural knowledge of the binding modes of VX-680, an Aurora kinase inhibitor, and BI 2536, a Polo-like kinase inhibitor, to design and evaluate drug-resistant kinase mutants. Using inducible stable human cell lines, we authenticated mitotic targets for both compounds and demonstrated that Aurora A mutants exhibit differential cellular sensitivity toward the inhibitors VX-680 and MLN8054. In addition, we validated Aurora B as an important anti-proliferative target for VX-680 in model human cancer cells. Finally, this chemical genetic approach allowed us to prove that Aurora A activation loop phosphorylation is controlled by a Plk1-mediated pathway in human cells.
Figures





References
-
- Cohen P. ( 2009) Curr. Opin. Cell Biol. 21, 317– 324 - PubMed
-
- Zhang J., Yang P. L., Gray N. S. ( 2009) Nat. Rev. Cancer 9, 28– 39 - PubMed
-
- Druker B. J. ( 2008) Blood 112, 4808– 4817 - PubMed
-
- Fabian M. A., Biggs W. H., 3rd, Treiber D. K., Atteridge C. E., Azimioara M. D., Benedetti M. G., Carter T. A., Ciceri P., Edeen P. T., Floyd M., Ford J. M., Galvin M., Gerlach J. L., Grotzfeld R. M., Herrgard S., Insko D. E., Insko M. A., Lai A. G., Lélias J. M., Mehta S. A., Milanov Z. V., Velasco A. M., Wodicka L. M., Patel H. K., Zarrinkar P. P., Lockhart D. J. ( 2005) Nat. Biotechnol. 23, 329– 336 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Chemical Information
Miscellaneous

