Reduced adiposity and high-fat diet-induced adipose inflammation in mice deficient for phosphodiesterase 4B
- PMID: 19359377
- PMCID: PMC2703511
- DOI: 10.1210/en.2009-0108
Reduced adiposity and high-fat diet-induced adipose inflammation in mice deficient for phosphodiesterase 4B
Abstract
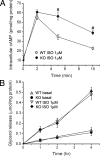
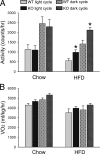
The concept that obesity is an inflammatory state has changed our understanding of this condition and suggested that pharmacological interventions targeting inflammation may be useful strategies to improve metabolic complications of obesity. Phosphodiesterase 4 (PDE4) inhibitors exhibit profound antiinflammatory effects, but whether PDE4 inhibition suppresses obesity-induced inflammation is unknown. Among PDE4 isoforms, PDE4B is the major species mediating inflammatory responses. We therefore examined obesity-related phenotypes in mice deficient for PDE4B. Compared with wild-type littermates, PDE4B-null mice were leaner, with lower fat pad weights, smaller adipocytes, and decreased serum leptin levels on both chow and high-fat diets (HFDs). PDE4B deficiency suppressed TNF-alpha mRNA levels and macrophage infiltration in white adipose tissue in mice on HFD, but insulin sensitivity was unaltered. PDE4B-null mice on HFDs had increased locomotor activity. These results suggest a previously unappreciated role for PDE4B in the regulation of energy balance and that PDE4B inhibitors could have utility in treatment of obesity and for suppression of obesity-induced inflammation in white adipose tissue.
Figures




Similar articles
-
Eicosapentaenoic acid reduces adipocyte hypertrophy and inflammation in diet-induced obese mice in an adiposity-independent manner.J Nutr. 2015 Mar;145(3):411-7. doi: 10.3945/jn.114.202952. Epub 2014 Dec 31. J Nutr. 2015. PMID: 25733455
-
Disruption of inducible 6-phosphofructo-2-kinase ameliorates diet-induced adiposity but exacerbates systemic insulin resistance and adipose tissue inflammatory response.J Biol Chem. 2010 Feb 5;285(6):3713-3721. doi: 10.1074/jbc.M109.058446. Epub 2009 Nov 30. J Biol Chem. 2010. PMID: 19948719 Free PMC article.
-
Obesity-induced inflammation in white adipose tissue is attenuated by loss of melanocortin-3 receptor signaling.Endocrinology. 2007 Dec;148(12):6186-94. doi: 10.1210/en.2007-0699. Epub 2007 Sep 27. Endocrinology. 2007. PMID: 17901224
-
Diet-induced obese mice are leptin insufficient after weight reduction.Obesity (Silver Spring). 2009 Sep;17(9):1702-9. doi: 10.1038/oby.2009.106. Epub 2009 Apr 16. Obesity (Silver Spring). 2009. PMID: 19373220 Free PMC article.
-
Phosphodiesterase 4B (PDE4B) inhibitors and their applications in recent years (2014 to early 2025).Mol Divers. 2025 Jun 14. doi: 10.1007/s11030-025-11242-2. Online ahead of print. Mol Divers. 2025. PMID: 40515964 Review.
Cited by
-
Roflumilast Suppresses Adipogenic Differentiation via AMPK Mediated Pathway.Front Endocrinol (Lausanne). 2021 Jun 7;12:662451. doi: 10.3389/fendo.2021.662451. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 34163436 Free PMC article.
-
Effects of Early-Life Stress, Postnatal Diet Modulation and Long-Term Western-Style Diet on Peripheral and Central Inflammatory Markers.Nutrients. 2021 Jan 20;13(2):288. doi: 10.3390/nu13020288. Nutrients. 2021. PMID: 33498469 Free PMC article.
-
Advances in targeting cyclic nucleotide phosphodiesterases.Nat Rev Drug Discov. 2014 Apr;13(4):290-314. doi: 10.1038/nrd4228. Nat Rev Drug Discov. 2014. PMID: 24687066 Free PMC article. Review.
-
The Other Side of the Perfect Cup: Coffee-Derived Non-Polyphenols and Their Roles in Mitigating Factors Affecting the Pathogenesis of Type 2 Diabetes.Int J Mol Sci. 2024 Aug 17;25(16):8966. doi: 10.3390/ijms25168966. Int J Mol Sci. 2024. PMID: 39201652 Free PMC article. Review.
-
Efficacy and Metabolic Effect on Serum Lipids of Apremilast in Psoriatic Arthritis: A Case Report.J Clin Med. 2019 Mar 22;8(3):398. doi: 10.3390/jcm8030398. J Clin Med. 2019. PMID: 30909370 Free PMC article.
References
-
- Taubes G 1998 As obesity rates rise, experts struggle to explain why. Science 280:1367–1368 - PubMed
-
- Popkin BM, Doak CM 1998 The obesity epidemic is a worldwide phenomenon. Nutr Rev 56:106–114 - PubMed
-
- Allison DB, Fontaine KR, Manson JE, Stevens J, VanItallie TB 1999 Annual deaths attributable to obesity in the United States. JAMA 282:1530–1538 - PubMed
-
- Billington CJ, Epstein LH, Goodwin NJ, Hill JO, Pi-Sunyer JX, Rolls BJ, Stern J, Wadden TA, Weinsier RL, Wilson GT, Wing RR, Yanovski SZ, Hubbard VS, Hoofnagle JH, Everhart J, Harrison B 2000 Overweight, obesity, and health risk. National Task Force on the Prevention and Treatment of Obesity. Arch Intern Med 160:898–904 - PubMed
-
- Spiegelman BM, Flier JS 2001 Obesity and the regulation of energy balance. Cell 104:531–543 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

