Subfertile female androgen receptor knockout mice exhibit defects in neuroendocrine signaling, intraovarian function, and uterine development but not uterine function
- PMID: 19359383
- PMCID: PMC2703552
- DOI: 10.1210/en.2008-1750
Subfertile female androgen receptor knockout mice exhibit defects in neuroendocrine signaling, intraovarian function, and uterine development but not uterine function
Abstract
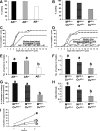
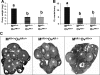
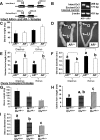
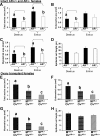
Female androgen receptor (AR) knockout mice (AR(-/-)) generated by an in-frame Ar exon 3 deletion are subfertile, but the mechanism is not clearly defined. To distinguish between extra- and intraovarian defects, reciprocal ovarian transplants were undertaken. Ovariectomized AR(-/-) hosts with wild-type (AR(+/+)) ovary transplants displayed abnormal estrus cycles, with longer cycles (50%, P < 0.05), and 66% were infertile (P < 0.05), whereas AR(+/+) hosts with either AR(-/-) or surgical control AR(+/+) ovary transplants displayed normal estrus cycles and fertility. These data imply a neuroendocrine defect, which is further supported by increased FSH (P <0.05) and estradiol (P <0.05), and greater LH suppressibility by estradiol in AR(-/-) females at estrus (P <0.05). Additional intraovarian defects were observed by the finding that both experimental transplant groups exhibited significantly reduced pups per litter (P < 0.05) and corpora lutea numbers (P < 0.05) compared with surgical controls. All groups exhibited normal uterine and lactation functions. AR(-/-) uteri were morphologically different from AR(+/+) with an increase in horn length (P < 0.01) but a reduction in uterine diameter (P < 0.05), total uterine area (P < 0.05), endometrial area (P < 0.05), and myometrial area (P < 0.01) at diestrus, indicating a role for AR in uterine growth and development. Both experimental transplant groups displayed a significant reduction in uterine diameter (P < 0.01) compared with transplanted wild-type controls, indicating a role for both AR-mediated intraovarian and intrauterine influences on uterine physiology. In conclusion, these data provide direct evidence that extraovarian neuroendocrine, but not uterine effects, as well as local intraovarian AR-mediated actions are important in maintaining female fertility, and a disruption of AR signaling leads to altered uterine development.
Figures

References
-
- Lubahn DB, Joseph DR, Sullivan PM, Willard HF, French FS, Wilson EM 1988 Cloning of human androgen receptor complementary DNA and localization to the X chromosome. Science 240:327–330 - PubMed
-
- Hillier SG, Whitelaw PF, Smyth CD 1994 Follicular oestrogen synthesis: the ‘two-cell, two-gonadotrophin’ model revisited. Mol Cell Endocrinol 100:51–54 - PubMed
-
- Walters KA, Allan CM, Jimenez M, Lim PR, Davey RA, Zajac JD, Illingworth P, Handelsman DJ 2007 Female mice haploinsufficient for an inactivated androgen receptor (AR) exhibit age-dependent defects that resemble the AR null phenotype of dysfunctional late follicle development, ovulation, and fertility. Endocrinology 148:3674–3684 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

