A novel Toll-like receptor 4 antagonist antibody ameliorates inflammation but impairs mucosal healing in murine colitis
- PMID: 19359427
- PMCID: PMC2697943
- DOI: 10.1152/ajpgi.90496.2008
A novel Toll-like receptor 4 antagonist antibody ameliorates inflammation but impairs mucosal healing in murine colitis
Abstract
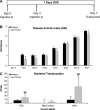
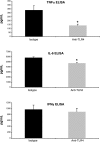
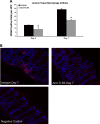
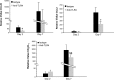
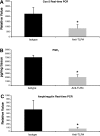
Dysregulated innate immune responses to commensal bacteria contribute to the development of inflammatory bowel disease (IBD). TLR4 is overexpressed in the intestinal mucosa of IBD patients and may contribute to uncontrolled inflammation. However, TLR4 is also an important mediator of intestinal repair. The aim of this study is to examine the effect of a TLR4 antagonist on inflammation and intestinal repair in two murine models of IBD. Colitis was induced in C57BL/6J mice with dextran sodium sulfate (DSS) or by transferring CD45Rb(hi) T cells into RAG1-/- mice. An antibody (Ab) against the TLR4/MD-2 complex or isotype control Ab was administered intraperitoneally during DSS treatment, recovery from DSS colitis, or induction of colitis in RAG1-/- mice. Colitis severity was assessed by disease activity index (DAI) and histology. The effect of the Ab on the inflammatory infiltrate was determined by cell isolation and immunohistochemistry. Mucosal expression of inflammatory mediators was analyzed by real-time PCR and ELISA. Blocking TLR4 at the beginning of DSS administration delayed the development of colitis with significantly lower DAI scores. Anti-TLR4 Ab treatment decreased macrophage and dendritic cell infiltrate and reduced mucosal expression of CCL2, CCL20, TNF-alpha, and IL-6. Anti-TLR4 Ab treatment during recovery from DSS colitis resulted in defective mucosal healing with lower expression of COX-2, PGE(2), and amphiregulin. In contrast, TLR4 blockade had minimal efficacy in ameliorating inflammation in the adoptive transfer model of chronic colitis. Our findings suggest that anti-TLR4 therapy may decrease inflammation in IBD but may also interfere with colonic mucosal healing.
Figures


References
-
- Aranda R, Sydora BC, McAllister PL, Binder SW, Yang HY, Targan SR, Kronenberg M. Analysis of intestinal lymphocytes in mouse colitis mediated by transfer of CD4+, CD45RBhigh T cells to SCID recipients. J Immunol 158: 3464–3473, 1997. - PubMed
-
- Bennett-Guerrero E, Grocott HP, Levy JH, Stierer KA, Hogue CW, Cheung AT, Newman MF, Carter AA, Rossignol DP, Collard CD. A phase II, double-blind, placebo-controlled, ascending-dose study of Eritoran (E5564), a lipid A antagonist, in patients undergoing cardiac surgery with cardiopulmonary bypass. Anesth Analg 104: 378–383, 2007. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

