Toll-like receptors as targets in chronic liver diseases
- PMID: 19359436
- PMCID: PMC2791673
- DOI: 10.1136/gut.2008.156307
Toll-like receptors as targets in chronic liver diseases
Abstract
Toll-like receptors (TLRs) recognise pathogen-associated molecular patterns (PAMPs) to detect the presence of pathogens. In addition to their role in innate immunity, TLRs also play a major role in the regulation of inflammation, even under sterile conditions such as injury and wound healing. This involvement has been suggested to depend, at least in part, on the ability of TLRs to recognise several endogenous TLR ligands termed damage-associated molecular patterns (DAMPs). The liver not only represents a major target of bacterial PAMPs in many disease states but also upregulates several DAMPs following injury. Accordingly, TLR-mediated signals have been implicated in a number of chronic liver diseases. Here, we will summarise recent findings on the role TLRs and TLR ligands in the pathophysiology of liver fibrosis and cirrhosis, viral hepatitis, alcoholic liver disease, non-alcoholic fatty liver disease and hepatocellular carcinoma, and highlight the potential role of TLR agonists, antagonists and probiotics for the treatment of chronic liver disease.
Figures
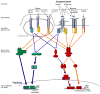
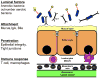
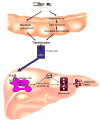
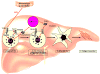
Comment in
-
Toll-like receptors 2/4 agonists: a potential strategy for preventing invasion and metastasis of hepatocellular carcinoma.Gut. 2010 Oct;59(10):1447-8; author reply 1448-9. doi: 10.1136/gut.2009.190835. Epub 2010 Jul 30. Gut. 2010. PMID: 20675699 No abstract available.
References
-
- Aderem A, Ulevitch RJ. Toll-like receptors in the induction of the innate immune response. Nature. 2000;406:782–7. - PubMed
-
- Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. - PubMed
-
- Lemaitre B, Nicolas E, Michaut L, et al. The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell. 1996;86:973–83. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
