IL-1 acts directly on CD4 T cells to enhance their antigen-driven expansion and differentiation
- PMID: 19359475
- PMCID: PMC2678417
- DOI: 10.1073/pnas.0902745106
IL-1 acts directly on CD4 T cells to enhance their antigen-driven expansion and differentiation
Abstract
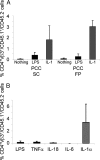
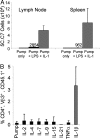
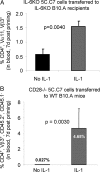
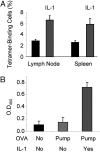
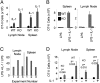
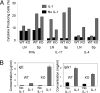
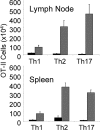
IL-1 causes a marked increase in the degree of expansion of naïve and memory CD4 T cells in response to challenge with their cognate antigen. The response occurs when only specific CD4 T cells can respond to IL-1beta, is not induced by a series of other cytokines and does not depend on IL-6 or CD-28. When WT cells are primed in IL-1R1(-/-) recipients, IL-1 increases the proportion of cytokine-producing transgenic CD4 T cells, especially IL-17- and IL-4-producing cells, strikingly increases serum IgE levels and serum IgG1 levels. IL-1beta enhances antigen-mediated expansion of in vitro primed Th1, Th2, and Th17 cells transferred to IL-1R1(-/-) recipients. The IL-1 receptor antagonist diminished responses to antigen plus lipopolysaccharide (LPS) by approximately 55%. These results indicate that IL-1beta signaling in T cells markedly induces robust and durable primary and secondary CD4 responses.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Joffre O, Nolte MA, Spörri R, Reis e Sousa C. Inflammatory signals in dendritic cell activation and the induction of adaptive immunity. Immunol Rev. 2009;227:234–247. - PubMed
-
- Dinarello CA. Interleukin-1 family of ligands and receptors. In: Paul WE, editor. Fundamental Immunology. 6th Ed. Philadelphia: Lippincott Williams & Wilkins;
-
- Chen Q, Sen G, Snapper CM. Endogenous IL-1R1 signaling is critical for cognate CD4+ T cell help for induction of in vivo type 1 and type 2 antipolysaccharide and antiprotein Ig isotype responses to intact Streptococcus pneumoniae, but not to a soluble pneumococcal conjugate vaccine. J Immunol. 2006;177:6044–6051. - PubMed
-
- Zwijnenburg PJ, et al. IL-1 receptor type 1 gene-deficient mice demonstrate an impaired host defense against pneumococcal meningitis. J Immunol. 2003;170:4724–4730. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

