Structural basis for cAMP-mediated allosteric control of the catabolite activator protein
- PMID: 19359484
- PMCID: PMC2678429
- DOI: 10.1073/pnas.0900595106
Structural basis for cAMP-mediated allosteric control of the catabolite activator protein
Abstract
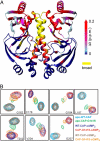
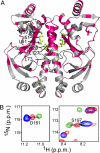
The cAMP-mediated allosteric transition in the catabolite activator protein (CAP; also known as the cAMP receptor protein, CRP) is a textbook example of modulation of DNA-binding activity by small-molecule binding. Here we report the structure of CAP in the absence of cAMP, which, together with structures of CAP in the presence of cAMP, defines atomic details of the cAMP-mediated allosteric transition. The structural changes, and their relationship to cAMP binding and DNA binding, are remarkably clear and simple. Binding of cAMP results in a coil-to-helix transition that extends the coiled-coil dimerization interface of CAP by 3 turns of helix and concomitantly causes rotation, by approximately 60 degrees , and translation, by approximately 7 A, of the DNA-binding domains (DBDs) of CAP, positioning the recognition helices in the DBDs in the correct orientation to interact with DNA. The allosteric transition is stabilized further by expulsion of an aromatic residue from the cAMP-binding pocket upon cAMP binding. The results define the structural mechanisms that underlie allosteric control of this prototypic transcriptional regulatory factor and provide an illustrative example of how effector-mediated structural changes can control the activity of regulatory proteins.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



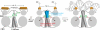
Comment in
-
Amplification of signaling via cellular allosteric relay and protein disorder.Proc Natl Acad Sci U S A. 2009 Apr 28;106(17):6887-8. doi: 10.1073/pnas.0903024106. Epub 2009 Apr 22. Proc Natl Acad Sci U S A. 2009. PMID: 19416924 Free PMC article. No abstract available.
References
-
- Martinez-Antonio A, Collado-Vides J. Identifying global regulators in transcriptional regulatory networks in bacteria. Current Opinion in Microbiology. 2003:482–489. - PubMed
-
- Benoff B, et al. Structural basis of transcription activation: The CAP-αCTD-DNA complex. Science. 2002;297:1562–1566. - PubMed
-
- Harman JG. Allosteric regulation of the cAMP receptor protein. Biochim Biophys Acta. 2001;1547:1–17. - PubMed
-
- McKay DB, Steitz TA. Structure of catabolite gene activator protein at 2.9 Å resolution suggests binding to left-handed B-DNA. Nature. 1981;290:744–749. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

