The bouquet of grapevine (Vitis vinifera L. cv. Cabernet Sauvignon) flowers arises from the biosynthesis of sesquiterpene volatiles in pollen grains
- PMID: 19359488
- PMCID: PMC2678416
- DOI: 10.1073/pnas.0901387106
The bouquet of grapevine (Vitis vinifera L. cv. Cabernet Sauvignon) flowers arises from the biosynthesis of sesquiterpene volatiles in pollen grains
Abstract
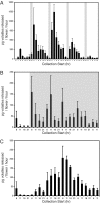
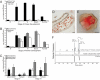
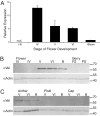
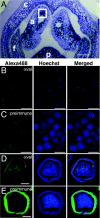
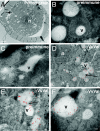
Terpenoid volatiles are important information molecules that enable pollinators to locate flowers and may protect reproductive tissues against pathogens or herbivores. Inflorescences of grapevine (Vitis vinifera L.) are composed of tiny green flowers that produce an abundance of sesquiterpenoid volatiles. We demonstrate that male flower parts of grapevines are responsible for sesquiterpenoid floral scent formation. We describe temporal and spatial patterns of biosynthesis and release of floral volatiles throughout the blooming of V. vinifera L. cv. Cabernet Sauvignon. The biosynthesis of sesquiterpene volatiles, which are emitted with a light-dependent diurnal pattern early in the morning at prebloom and bloom, is localized to anthers and, more specifically, within the developing pollen grains. Valencene synthase (VvValCS) enzyme activity, which produces the major sesquiterpene volatiles of grapevine flowers, is present in anthers. VvValCS transcripts are most abundant in flowers at prebloom stages. Western blot analysis identified VvValCS protein in anthers, and in situ immunolabeling located VvValCS protein in pollen grains during bloom. Histochemical staining, as well as immunolabeling analysis by fluorescent microscopy and transmission electron microscopy, indicated that VvValCS localizes close to lipid bodies within the maturing microspore.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Vitis vinifera terpenoid cyclases: functional identification of two sesquiterpene synthase cDNAs encoding (+)-valencene synthase and (-)-germacrene D synthase and expression of mono- and sesquiterpene synthases in grapevine flowers and berries.Phytochemistry. 2004 Oct;65(19):2649-59. doi: 10.1016/j.phytochem.2004.08.017. Phytochemistry. 2004. PMID: 15464152
-
Biosynthesis and emission of terpenoid volatiles from Arabidopsis flowers.Plant Cell. 2003 Feb;15(2):481-94. doi: 10.1105/tpc.007989. Plant Cell. 2003. PMID: 12566586 Free PMC article.
-
Two sesquiterpene synthases are responsible for the complex mixture of sesquiterpenes emitted from Arabidopsis flowers.Plant J. 2005 Jun;42(5):757-71. doi: 10.1111/j.1365-313X.2005.02417.x. Plant J. 2005. PMID: 15918888
-
Understanding the Constitutive and Induced Biosynthesis of Mono- and Sesquiterpenes in Grapes (Vitis vinifera): A Key to Unlocking the Biochemical Secrets of Unique Grape Aroma Profiles.J Agric Food Chem. 2015 Dec 16;63(49):10591-603. doi: 10.1021/acs.jafc.5b04398. Epub 2015 Dec 3. J Agric Food Chem. 2015. PMID: 26592256 Review.
-
Sugars and flowering in the grapevine (Vitis vinifera L.).J Exp Bot. 2008;59(10):2565-78. doi: 10.1093/jxb/ern135. Epub 2008 May 28. J Exp Bot. 2008. PMID: 18508810 Review.
Cited by
-
Grapevine mono- and sesquiterpenes: Genetics, metabolism, and ecophysiology.Front Plant Sci. 2023 Feb 3;14:1111392. doi: 10.3389/fpls.2023.1111392. eCollection 2023. Front Plant Sci. 2023. PMID: 36818850 Free PMC article. Review.
-
Functional annotation, genome organization and phylogeny of the grapevine (Vitis vinifera) terpene synthase gene family based on genome assembly, FLcDNA cloning, and enzyme assays.BMC Plant Biol. 2010 Oct 21;10:226. doi: 10.1186/1471-2229-10-226. BMC Plant Biol. 2010. PMID: 20964856 Free PMC article.
-
Annotation, classification, genomic organization and expression of the Vitis vinifera CYPome.PLoS One. 2018 Jun 28;13(6):e0199902. doi: 10.1371/journal.pone.0199902. eCollection 2018. PLoS One. 2018. PMID: 29953551 Free PMC article.
-
Untargeted flower volatilome profiling highlights differential pollinator attraction strategies in muscadine.Front Plant Sci. 2025 Feb 28;16:1548564. doi: 10.3389/fpls.2025.1548564. eCollection 2025. Front Plant Sci. 2025. PMID: 40093614 Free PMC article.
-
Variation of herbivore-induced volatile terpenes among Arabidopsis ecotypes depends on allelic differences and subcellular targeting of two terpene synthases, TPS02 and TPS03.Plant Physiol. 2010 Jul;153(3):1293-310. doi: 10.1104/pp.110.154864. Epub 2010 May 12. Plant Physiol. 2010. PMID: 20463089 Free PMC article.
References
-
- Dobson HEM, Bergstroem G. The ecology and evolution of pollen odors. Plant Syst Evol. 2000;222:63–87.
-
- Pellmyr O, Thien LB. Insect reproduction and floral fragrances. Taxon. 1986;35:76–85.
-
- Ashman T-L, Cole DH, Bradburn M, Blaney B, Raguso RA. The scent of a male. Ecology. 2005;86:2099–2105.
-
- Pacini E, Hesse M. Pollenkitt: Its composition, forms and functions. Flora (Jena) 2005;200:399–415.
-
- Piffanelli P, Ross JHE, Murphy DJ. Biogenesis and function of the lipidic structures of pollen grains. Sex Plant Reprod. 1998;11:65–80.
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

