Positive role of CCAAT/enhancer-binding protein homologous protein, a transcription factor involved in the endoplasmic reticulum stress response in the development of colitis
- PMID: 19359519
- PMCID: PMC2671267
- DOI: 10.2353/ajpath.2009.080864
Positive role of CCAAT/enhancer-binding protein homologous protein, a transcription factor involved in the endoplasmic reticulum stress response in the development of colitis
Abstract
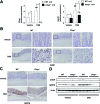
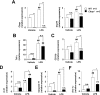
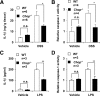
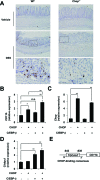
Although recent reports suggest that the endoplasmic reticulum (ER) stress response is induced in association with the development of inflammatory bowel disease, its role in the pathogenesis of inflammatory bowel disease remains unclear. The CCAAT/enhancer-binding protein (C/EBP) homologous protein (CHOP) is a transcription factor that is involved in the ER stress response, especially ER stress-induced apoptosis. In this study, we found that experimental colitis was ameliorated in CHOP-null mice, suggesting that CHOP exacerbates the development of colitis. The mRNA expression of Mac-1 (CD11b, a positive regulator of macrophage infiltration), Ero-1alpha, and Caspase-11 (a positive regulator of interleukin-1beta production) in the intestine was induced with the development of colitis, and this induction was suppressed in CHOP-null mice. ERO-1alpha is involved in the production of reactive oxygen species (ROS); an increase in ROS production, which is associated with the development of colitis in the intestine, was suppressed in CHOP-null mice. A greater number of apoptotic cells in the intestinal mucosa of wild-type mice were observed to accompany the development of colitis compared with CHOP-null mice, suggesting that up-regulation of CHOP expression exacerbates the development of colitis. Furthermore, this CHOP activity appears to involve various stimulatory mechanisms, such as macrophage infiltration via the induction of Mac-1, ROS production via the induction of ERO-1alpha, interleukin-1beta production via the induction of Caspase-11, and intestinal mucosal cell apoptosis.
Figures





Similar articles
-
CCAAT-Enhancer-Binding Protein Homologous Protein Deficiency Attenuates Oxidative Stress and Renal Ischemia-Reperfusion Injury.Antioxid Redox Signal. 2015 Nov 20;23(15):1233-45. doi: 10.1089/ars.2013.5768. Epub 2014 Oct 17. Antioxid Redox Signal. 2015. PMID: 25178318
-
Induction of apoptosis by cigarette smoke via ROS-dependent endoplasmic reticulum stress and CCAAT/enhancer-binding protein-homologous protein (CHOP).Free Radic Biol Med. 2008 Jul 1;45(1):50-9. doi: 10.1016/j.freeradbiomed.2008.03.003. Epub 2008 Mar 18. Free Radic Biol Med. 2008. PMID: 18394432
-
Interleukin-1α deficiency attenuates endoplasmic reticulum stress-induced liver damage and CHOP expression in mice.J Hepatol. 2015 Oct;63(4):926-33. doi: 10.1016/j.jhep.2015.05.012. Epub 2015 May 27. J Hepatol. 2015. PMID: 26022690
-
New insights into the roles of CHOP-induced apoptosis in ER stress.Acta Biochim Biophys Sin (Shanghai). 2014 Aug;46(8):629-40. doi: 10.1093/abbs/gmu048. Acta Biochim Biophys Sin (Shanghai). 2014. PMID: 25016584 Review.
-
A crosstalk between p21 and UPR-induced transcription factor C/EBP homologous protein (CHOP) linked to type 2 diabetes.Biochimie. 2014 Apr;99:19-27. doi: 10.1016/j.biochi.2013.11.003. Epub 2013 Nov 14. Biochimie. 2014. PMID: 24239558 Review.
Cited by
-
Toll-like receptors in inflammatory bowel diseases: a decade later.Inflamm Bowel Dis. 2010 Sep;16(9):1583-97. doi: 10.1002/ibd.21282. Inflamm Bowel Dis. 2010. PMID: 20803699 Free PMC article. Review.
-
Protein Kinase R in Bacterial Infections: Friend or Foe?Front Immunol. 2021 Jul 8;12:702142. doi: 10.3389/fimmu.2021.702142. eCollection 2021. Front Immunol. 2021. PMID: 34305942 Free PMC article. Review.
-
Villin-1 and Gelsolin Regulate Changes in Actin Dynamics That Affect Cell Survival Signaling Pathways and Intestinal Inflammation.Gastroenterology. 2018 Apr;154(5):1405-1420.e2. doi: 10.1053/j.gastro.2017.12.016. Epub 2017 Dec 21. Gastroenterology. 2018. PMID: 29274870 Free PMC article.
-
Gut bacterial metabolites modulate endoplasmic reticulum stress.Genome Biol. 2021 Oct 15;22(1):292. doi: 10.1186/s13059-021-02496-8. Genome Biol. 2021. PMID: 34654459 Free PMC article.
-
PKR-dependent CHOP induction limits hyperoxia-induced lung injury.Am J Physiol Lung Cell Mol Physiol. 2011 Mar;300(3):L422-9. doi: 10.1152/ajplung.00166.2010. Epub 2010 Dec 24. Am J Physiol Lung Cell Mol Physiol. 2011. PMID: 21186267 Free PMC article.
References
-
- Cuzzocrea S. Emerging biotherapies for inflammatory bowel disease. Expert Opin Emerg Drugs. 2003;8:339–347. - PubMed
-
- Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417–429. - PubMed
-
- Jurjus AR, Khoury NN, Reimund JM. Animal models of inflammatory bowel disease. J Pharmacol Toxicol Methods. 2004;50:81–92. - PubMed
-
- Danese S, Semeraro S, Marini M, Roberto I, Armuzzi A, Papa A, Gasbarrini A. Adhesion molecules in inflammatory bowel disease: therapeutic implications for gut inflammation. Dig Liver Dis. 2005;37:811–818. - PubMed
-
- Reinecker HC, Steffen M, Witthoeft T, Pflueger I, Schreiber S, MacDermott RP, Raedler A. Enhanced secretion of tumour necrosis factor-alpha, IL-6, and IL-1 beta by isolated lamina propria mononuclear cells from patients with ulcerative colitis and Crohn’s disease. Clin Exp Immunol. 1993;94:174–181. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

