TRPV4 channels mediate cyclic strain-induced endothelial cell reorientation through integrin-to-integrin signaling
- PMID: 19359599
- PMCID: PMC2754067
- DOI: 10.1161/CIRCRESAHA.108.192930
TRPV4 channels mediate cyclic strain-induced endothelial cell reorientation through integrin-to-integrin signaling
Abstract
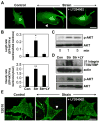
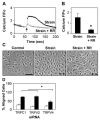
Cyclic mechanical strain produced by pulsatile blood flow regulates the orientation of endothelial cells lining blood vessels and influences critical processes such as angiogenesis. Mechanical stimulation of stretch-activated calcium channels is known to mediate this reorientation response; however, the molecular basis remains unknown. Here, we show that cyclically stretching capillary endothelial cells adherent to flexible extracellular matrix substrates activates mechanosensitive TRPV4 (transient receptor potential vanilloid 4) ion channels that, in turn, stimulate phosphatidylinositol 3-kinase-dependent activation and binding of additional beta1 integrin receptors, which promotes cytoskeletal remodeling and cell reorientation. Inhibition of integrin activation using blocking antibodies and knock down of TRPV4 channels using specific small interfering RNA suppress strain-induced capillary cell reorientation. Thus, mechanical forces that physically deform extracellular matrix may guide capillary cell reorientation through a strain-dependent "integrin-to-integrin" signaling mechanism mediated by force-induced activation of mechanically gated TRPV4 ion channels on the cell surface.
Figures
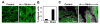



References
-
- Chien S, Li S, Shyy YJ. Effects of mechanical forces on signal transduction and gene expression in endothelial cells. Hypertension. 1998;31(1 Pt 2):162–169. - PubMed
-
- Ingber DE. Mechanical signaling and the cellular response to extracellular matrix in angiogenesis and cardiovascular physiology. Circ Res. 2002;91(10):877–887. - PubMed
-
- Davies PF, Mundel T, Barbee KA. A mechanism for heterogeneous endothelial responses to flow in vivo and in vitro. J Biomech. 1995;28(12):1553–1560. - PubMed
-
- Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Geometric control of cell life and death. Science. 1997;276(5317):1425–1428. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

