Positive feedback between transcriptional and kinase suppression in nematodes with extraordinary longevity and stress resistance
- PMID: 19360094
- PMCID: PMC2661368
- DOI: 10.1371/journal.pgen.1000452
Positive feedback between transcriptional and kinase suppression in nematodes with extraordinary longevity and stress resistance
Abstract
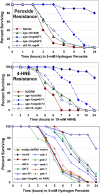
Insulin/IGF-1 signaling (IIS) regulates development and metabolism, and modulates aging, of Caenorhabditis elegans. In nematodes, as in mammals, IIS is understood to operate through a kinase-phosphorylation cascade that inactivates the DAF-16/FOXO transcription factor. Situated at the center of this pathway, phosphatidylinositol 3-kinase (PI3K) phosphorylates PIP(2) to form PIP(3), a phospholipid required for membrane tethering and activation of many signaling molecules. Nonsense mutants of age-1, the nematode gene encoding the class-I catalytic subunit of PI3K, produce only a truncated protein lacking the kinase domain, and yet confer 10-fold greater longevity on second-generation (F2) homozygotes, and comparable gains in stress resistance. Their F1 parents, like weaker age-1 mutants, are far less robust-implying that maternally contributed trace amounts of PI3K activity or of PIP(3) block the extreme age-1 phenotypes. We find that F2-mutant adults have <10% of wild-type kinase activity in vitro and <60% of normal phosphoprotein levels in vivo. Inactivation of PI3K not only disrupts PIP(3)-dependent kinase signaling, but surprisingly also attenuates transcripts of numerous IIS components, even upstream of PI3K, and those of signaling molecules that cross-talk with IIS. The age-1(mg44) nonsense mutation results, in F2 adults, in changes to kinase profiles and to expression levels of multiple transcripts that distinguish this mutant from F1 age-1 homozygotes, a weaker age-1 mutant, or wild-type adults. Most but not all of those changes are reversed by a second mutation to daf-16, implicating both DAF-16/ FOXO-dependent and -independent mechanisms. RNAi, silencing genes that are downregulated in long-lived worms, improves oxidative-stress resistance of wild-type adults. It is therefore plausible that attenuation of those genes in age-1(mg44)-F2 adults contributes to their exceptional survival. IIS in nematodes (and presumably in other species) thus involves transcriptional as well as kinase regulation in a positive-feedback circuit, favoring either survival or reproduction. Hyperlongevity of strong age-1(mg44) mutants may result from their inability to reset this molecular switch to the reproductive mode.
Conflict of interest statement
Three of the authors (CT, AS and RJSR) are listed as the inventors in a patent filed by the University of Arkansas for Medical Sciences, relating to novel aspects of second-generation homozygotes for the age-1(mg44) and (m333) mutant alleles, underlying their extreme longevity and stress resistance.
Figures



Similar articles
-
Caenorhabditis elegans PI3K mutants reveal novel genes underlying exceptional stress resistance and lifespan.Aging Cell. 2009 Dec;8(6):706-25. doi: 10.1111/j.1474-9726.2009.00524.x. Epub 2009 Sep 17. Aging Cell. 2009. PMID: 19764929 Free PMC article.
-
Activated AKT/PKB signaling in C. elegans uncouples temporally distinct outputs of DAF-2/insulin-like signaling.BMC Dev Biol. 2006 Oct 4;6:45. doi: 10.1186/1471-213X-6-45. BMC Dev Biol. 2006. PMID: 17020605 Free PMC article.
-
SHC-1/p52Shc targets the insulin/IGF-1 and JNK signaling pathways to modulate life span and stress response in C. elegans.Genes Dev. 2008 Oct 1;22(19):2721-35. doi: 10.1101/gad.478408. Genes Dev. 2008. PMID: 18832074 Free PMC article.
-
Extreme-longevity mutations orchestrate silencing of multiple signaling pathways.Biochim Biophys Acta. 2009 Oct;1790(10):1075-83. doi: 10.1016/j.bbagen.2009.05.011. Epub 2009 May 22. Biochim Biophys Acta. 2009. PMID: 19465083 Free PMC article. Review.
-
DAF-16/FoxO in Caenorhabditis elegans and Its Role in Metabolic Remodeling.Cells. 2020 Jan 2;9(1):109. doi: 10.3390/cells9010109. Cells. 2020. PMID: 31906434 Free PMC article. Review.
Cited by
-
Neuronal inputs and outputs of aging and longevity.Front Genet. 2013 May 6;4:71. doi: 10.3389/fgene.2013.00071. eCollection 2013. Front Genet. 2013. PMID: 23653632 Free PMC article.
-
Extreme Depletion of PIP3 Accompanies the Increased Life Span and Stress Tolerance of PI3K-null C. elegans Mutants.Front Genet. 2013 Mar 28;4:34. doi: 10.3389/fgene.2013.00034. eCollection 2013. Front Genet. 2013. PMID: 23543623 Free PMC article.
-
A recent global selective sweep on the age-1 phosphatidylinositol 3-OH kinase regulator of the insulin-like signaling pathway within Caenorhabditis remanei.G3 (Bethesda). 2014 Apr 11;4(6):1123-33. doi: 10.1534/g3.114.010629. G3 (Bethesda). 2014. PMID: 24727287 Free PMC article.
-
Comparison of the mammalian insulin signalling pathway to invertebrates in the context of FOXO-mediated ageing.Bioinformatics. 2014 Nov 1;30(21):2999-3003. doi: 10.1093/bioinformatics/btu493. Epub 2014 Jul 26. Bioinformatics. 2014. PMID: 25064569 Free PMC article.
-
A narrow quantitative trait locus in C. elegans coordinately affects longevity, thermotolerance, and resistance to paraquat.Front Genet. 2011 Sep 27;2:63. doi: 10.3389/fgene.2011.00063. eCollection 2011. Front Genet. 2011. PMID: 22303358 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

