Newly generated heparanase knock-out mice unravel co-regulation of heparanase and matrix metalloproteinases
- PMID: 19360105
- PMCID: PMC2664924
- DOI: 10.1371/journal.pone.0005181
Newly generated heparanase knock-out mice unravel co-regulation of heparanase and matrix metalloproteinases
Abstract
Background: Heparanase, a mammalian endo-beta-D-glucuronidase, specifically degrades heparan sulfate proteoglycans ubiquitously associated with the cell surface and extracellular matrix. This single gene encoded enzyme is over-expressed in most human cancers, promoting tumor metastasis and angiogenesis.
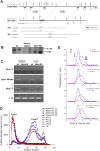
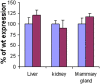
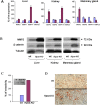
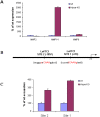
Principal findings: We report that targeted disruption of the murine heparanase gene eliminated heparanase enzymatic activity, resulting in accumulation of long heparan sulfate chains. Unexpectedly, the heparanase knockout (Hpse-KO) mice were fertile, exhibited a normal life span and did not show prominent pathological alterations. The lack of major abnormalities is attributed to a marked elevation in the expression of matrix metalloproteinases, for example, MMP2 and MMP14 in the Hpse-KO liver and kidney. Co-regulation of heparanase and MMPs was also noted by a marked decrease in MMP (primarily MMP-2,-9 and 14) expression following transfection and over-expression of the heparanase gene in cultured human mammary carcinoma (MDA-MB-231) cells. Immunostaining (kidney tissue) and chromatin immunoprecipitation (ChIP) analysis (Hpse-KO mouse embryonic fibroblasts) suggest that the newly discovered co-regulation of heparanase and MMPs is mediated by stabilization and transcriptional activity of beta-catenin.
Conclusions/significance: The lack of heparanase expression and activity was accompanied by alterations in the expression level of MMP family members, primarily MMP-2 and MMP-14. It is conceivable that MMP-2 and MMP-14, which exert some of the effects elicited by heparanase (i.e., over branching of mammary glands, enhanced angiogenic response) can compensate for its absence, in spite of their different enzymatic substrate. Generation of viable Hpse-KO mice lacking significant abnormalities may provide a promising indication for the use of heparanase as a target for drug development.
Conflict of interest statement
Figures



References
-
- Bernfield M, Gotte M, Park PW, Reizes O, Fitzgerald ML, et al. Functions of cell surface heparan sulfate proteoglycans. Annu Rev Biochem. 1999;68:729–777. - PubMed
-
- Kjellen L, Lindahl U. Proteoglycans: structures and interactions [published erratum appears in Annu Rev Biochem 1992;61:following viii]. Annu Rev Biochem. 1991;60:443–475. - PubMed
-
- Vlodavsky I, Bar-Shavit R, Korner G, Fuks Z. Extracellular matix-bound growth factors, enzymes and plasma proteins. In: Rohrbach DH, Timpl R, editors. Academic press Inc., editor. Basement membranes: Cellular and molecular aspects. Orlando, , Fl: 1993. pp. 327–343.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

