The NOD/RIP2 pathway is essential for host defenses against Chlamydophila pneumoniae lung infection
- PMID: 19360122
- PMCID: PMC2660273
- DOI: 10.1371/journal.ppat.1000379
The NOD/RIP2 pathway is essential for host defenses against Chlamydophila pneumoniae lung infection
Erratum in
- PLoS Pathog. 2009 Apr;5(4). doi: 10.1371/annotation/0f4b8a96-1598-4c90-b816-8fbe7614310b
- PLoS Pathog. 2009 Apr;5(4). doi: 10.1371/annotation/f3aa682e-3bc2-4a05-ac7f-05c6cfe1bbd7
Abstract
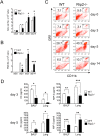
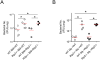
Here we investigated the role of the Nod/Rip2 pathway in host responses to Chlamydophila pneumoniae-induced pneumonia in mice. Rip2(-/-) mice infected with C. pneumoniae exhibited impaired iNOS expression and NO production, and delayed neutrophil recruitment to the lungs. Levels of IL-6 and IFN-gamma levels as well as KC and MIP-2 levels in bronchoalveolar lavage fluid (BALF) were significantly decreased in Rip2(-/-) mice compared to wild-type (WT) mice at day 3. Rip2(-/-) mice showed significant delay in bacterial clearance from the lungs and developed more severe and chronic lung inflammation that continued even on day 35 and led to increased mortality, whereas WT mice cleared the bacterial load, recovered from acute pneumonia, and survived. Both Nod1(-/-) and Nod2(-/-) mice also showed delayed bacterial clearance, suggesting that C. pneumoniae is recognized by both of these intracellular receptors. Bone marrow chimera experiments demonstrated that Rip2 in BM-derived cells rather than non-hematopoietic stromal cells played a key role in host responses in the lungs and clearance of C. pneumoniae. Furthermore, adoptive transfer of WT macrophages intratracheally was able to rescue the bacterial clearance defect in Rip2(-/-) mice. These results demonstrate that in addition to the TLR/MyD88 pathway, the Nod/Rip2 signaling pathway also plays a significant role in intracellular recognition, innate immune host responses, and ultimately has a decisive impact on clearance of C. pneumoniae from the lungs and survival of the infectious challenge.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





References
-
- Karvonen M, Tuomilehto J, Pitkäniemi J, Naukkarinen A, Saikku P. Chlamydia pneumoniae IgG antibody prevalence in south-western and eastern Finland in 1982 and 1987. Int J Epidemiol. 1994;23:176–184. - PubMed
-
- Campbell LA, Kuo CC. Chlamydia pneumoniae—an infectious risk factor for atherosclerosis? Nat Rev Mirobiol. 2004;2:23–32. - PubMed
-
- Hansbro PM, Beagley KW, Horvat JC, Gibson PG. Role of atypical bacterial infection of the lung in predisposition/protection of asthma. Pharmacol Ther. 2004;101:193–210. - PubMed
-
- Belland RJ, Ouellette SP, Gieffers J, Byrne GI. Chlamydia pneumoniae and atherosclerosis. Cell Microbiol. 2004;6:117–127. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

