Peripheral neuropathy in mice with neuronal nitric oxide synthase gene deficiency
- PMID: 19360314
- PMCID: PMC2756471
- DOI: 10.3892/ijmm_00000166
Peripheral neuropathy in mice with neuronal nitric oxide synthase gene deficiency
Abstract
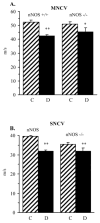
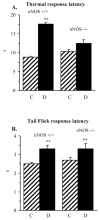
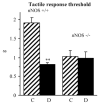
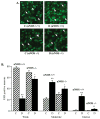
Evidence for the important role of the potent oxidant peroxynitrite in peripheral diabetic neuropathy and neuropathic pain is emerging. This study evaluated the contribution of neuronal nitric oxide synthase (nNOS) to diabetes-induced nitrosative stress in peripheral nerve and dorsal root ganglia, and peripheral nerve dysfunction and degeneration. Control and nNOS-/- mice were made diabetic with streptozotocin, and maintained for 6 weeks. Peroxynitrite injury was assessed by nitrotyrosine and poly(ADP-ribose) immunoreactivities. Peripheral diabetic neuropathy was evaluated by measurements of sciatic motor and hind-limb digital sensory nerve conduction velocities, thermal algesia, tactile allodynia, and intraepidermal nerve fiber density. Control nNOS-/- mice displayed normal motor nerve conduction velocity and thermal response latency, whereas sensory nerve conduction velocity was slightly lower compared with non-diabetic wild-type mice, and tactile response threshold and intraepidermal nerve fiber density were reduced by 47 and 38%, respectively. Both diabetic wild-type and nNOS-/- mice displayed enhanced nitrosative stress in peripheral nerve. In contrast to diabetic wild-type mice, diabetic nNOS-/- mice had near normal nitrotyrosine and poly(ADP-ribose) immunofluorescence in dorsal root ganglia. Both diabetic wild-type and nNOS-/- mice developed motor and sensory nerve conduction velocity deficits and thermal hypoalgesia although nNOS gene deficiency slightly reduced severity of the three disorders. Tactile response thresholds were similarly decreased in control and diabetic nNOS-/- mice compared with non-diabetic wild-type mice. Intraepidermal nerve fiber density was lower by 27% in diabetic nNOS-/- mice compared with the corresponding non-diabetic group, and by 20% in diabetic nNOS-/- mice compared with diabetic wild-type mice. In conclusion, nNOS is required for maintaining the normal peripheral nerve function and small sensory nerve fibre innervation. nNOS gene deficiency does not protect from development of nerve conduction deficit, sensory neuropathy and intraepidermal nerve fiber loss.
Figures




Similar articles
-
Inducible nitric oxide synthase gene deficiency counteracts multiple manifestations of peripheral neuropathy in a streptozotocin-induced mouse model of diabetes.Diabetologia. 2008 Nov;51(11):2126-33. doi: 10.1007/s00125-008-1136-3. Epub 2008 Sep 19. Diabetologia. 2008. PMID: 18802679 Free PMC article.
-
A peroxynitrite decomposition catalyst counteracts sensory neuropathy in streptozotocin-diabetic mice.Eur J Pharmacol. 2007 Aug 13;569(1-2):48-58. doi: 10.1016/j.ejphar.2007.05.055. Epub 2007 Jun 9. Eur J Pharmacol. 2007. PMID: 17644085 Free PMC article.
-
Nitrosative stress and peripheral diabetic neuropathy in leptin-deficient (ob/ob) mice.Exp Neurol. 2007 Jun;205(2):425-36. doi: 10.1016/j.expneurol.2007.03.019. Epub 2007 Mar 27. Exp Neurol. 2007. PMID: 17475250
-
Evaluation of the peroxynitrite decomposition catalyst Fe(III) tetra-mesitylporphyrin octasulfonate on peripheral neuropathy in a mouse model of type 1 diabetes.Int J Mol Med. 2007 Dec;20(6):783-92. Int J Mol Med. 2007. PMID: 17982684 Free PMC article.
-
Epidermal nerve fiber quantification in the assessment of diabetic neuropathy.Acta Histochem. 2008;110(5):351-62. doi: 10.1016/j.acthis.2007.12.004. Epub 2008 Apr 1. Acta Histochem. 2008. PMID: 18384843 Free PMC article. Review.
Cited by
-
Salvianolic acid A protects the peripheral nerve function in diabetic rats through regulation of the AMPK-PGC1α-Sirt3 axis.Molecules. 2012 Sep 20;17(9):11216-28. doi: 10.3390/molecules170911216. Molecules. 2012. PMID: 22996345 Free PMC article.
-
Dysregulation of nitric oxide synthases during early and late pathophysiological conditions of diabetes mellitus leads to amassing of microvascular impedement.J Diabetes Metab Disord. 2021 Apr 21;20(1):989-1002. doi: 10.1007/s40200-021-00799-y. eCollection 2021 Jun. J Diabetes Metab Disord. 2021. PMID: 34178871 Free PMC article. Review.
-
The expanding roles of neuronal nitric oxide synthase (NOS1).PeerJ. 2022 Jul 7;10:e13651. doi: 10.7717/peerj.13651. eCollection 2022. PeerJ. 2022. PMID: 35821897 Free PMC article. Review.
-
Oxidative and nitrative stress in neurodegeneration.Neurobiol Dis. 2015 Dec;84:4-21. doi: 10.1016/j.nbd.2015.04.020. Epub 2015 May 27. Neurobiol Dis. 2015. PMID: 26024962 Free PMC article. Review.
-
Diabetic painful and insensate neuropathy: pathogenesis and potential treatments.Neurotherapeutics. 2009 Oct;6(4):638-47. doi: 10.1016/j.nurt.2009.07.004. Neurotherapeutics. 2009. PMID: 19789069 Free PMC article. Review.
References
-
- Virág L, Szabó E, Gergely P, Szabó C. Peroxynitrite-induced cytotoxicity: mechanism and opportunities for intervention. Toxicol Lett. 2003;140–141:113–124. - PubMed
-
- Szabó C, Ischiropoulos H, Radi R. Peroxynitrite: biochemistry, pathophysiology and development of therapeutics. Nat Rev Drug Discov. 2007;6:662–680. - PubMed
-
- Szabó C, Mabley JG, Moeller SM, Shimanovich R, Pacher P, Virag L, Soriano FG, van Duzer JH, Williams W, Salzman AL, Groves JT. Part I. Pathogenetic role of peroxynitrite in the development of diabetes and diabetic vascular complications: studies with FP15, a novel potent peroxynitrite decomposition catalyst. Mol Med. 2002;8:571–580. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

