Chemical approaches to perturb, profile, and perceive glycans
- PMID: 19361192
- PMCID: PMC2697281
- DOI: 10.1021/ar800267j
Chemical approaches to perturb, profile, and perceive glycans
Abstract
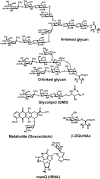
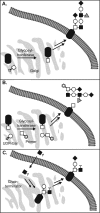
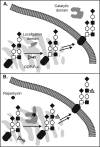
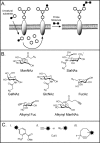
Glycosylation is an essential form of post-translational modification that regulates intracellular and extracellular processes. Regrettably, conventional biochemical and genetic methods often fall short for the study of glycans, because their structures are often not precisely defined at the genetic level. To address this deficiency, chemists have developed technologies to perturb glycan biosynthesis, profile their presentation at the systems level, and perceive their spatial distribution. These tools have identified potential disease biomarkers and ways to monitor dynamic changes to the glycome in living organisms. Still, glycosylation remains the underexplored frontier of many biological systems. In this Account, we focus on research in our laboratory that seeks to transform the study of glycan function from a challenge to routine practice. In studies of proteins and nucleic acids, functional studies have often relied on genetic manipulations to perturb structure. Though not directly subject to mutation, we can determine glycan structure-function relationships by synthesizing defined glycoconjugates or by altering natural glycosylation pathways. Chemical syntheses of uniform glycoproteins and polymeric glycoprotein mimics have facilitated the study of individual glycoconjugates in the absence of glycan microheterogeneity. Alternatively, selective inhibition or activation of glycosyltransferases or glycosidases can define the biological roles of the corresponding glycans. Investigators have developed tools including small molecule inhibitors, decoy substrates, and engineered proteins to modify cellular glycans. Current approaches offer a precision approaching that of genetic control. Genomic and proteomic profiling form a basis for biological discovery. Glycans also present a rich matrix of information that adapts rapidly to changing environs. Glycomic and glycoproteomic analyses via microarrays and mass spectrometry are beginning to characterize alterations in glycans that correlate with disease. These approaches have already identified several cancer biomarkers. Metabolic labeling can identify recently synthesized glycans and thus directly track glycan dynamics. This approach can highlight changes in physiology or environment and may be more informative than steady-state analyses. Together, glycomic and metabolic labeling techniques provide a comprehensive description of glycosylation as a foundation for hypothesis generation. Direct visualization of proteins via the green fluorescent protein (GFP) and its congeners has revolutionized the field of protein dynamics. Similarly, the ability to perceive the spatial organization of glycans could transform our understanding of their role in development, infection, and disease progression. Fluorescent tagging in cultured cells and developing organisms has revealed important insights into the dynamics of these structures during growth and development. These results have highlighted the need for additional imaging probes.
Figures


References
-
- Apweiler R.; Hermjakob H.; Sharon N. On the frequency of protein glycosylation, as deduced from analysis of the SWISS-PROT database. Biochim. Biophys. Acta 1999, 1473, 4–8. - PubMed
-
- Jung E.; Veuthey A. L.; Gasteiger E.; Bairoch A. Annotation of glycoproteins in the SWISS-PROT database. Proteomics 2001, 1, 262–268. - PubMed
-
- Kasai H.; Nakanishi K.; Macfarlane R. D.; Torgerson D. F.; Ohashi Z.; McCloskey J. A.; Gross H. J.; Nishimura S. The structure of Q* nucleoside isolated from rabbit liver transfer ribonucleic acid. J. Am. Chem. Soc. 1976, 98, 5044–5046. - PubMed
-
- Mitra N.; Sharon N.; Surolia A. Role of N-linked glycan in the unfolding pathway of Erythrina corallodendron lectin. Biochemistry 2003, 42, 12208–12216. - PubMed
-
- Grobe K.; Ledin J.; Ringvall M.; Holmborn K.; Forsberg E.; Esko J. D.; Kjellen L. Heparan sulfate and development: Differential roles of the N-acetylglucosamine N-deacetylase N-sulfotransferase isozymes. Biochim. Biophys. Acta 2002, 1573, 209–215. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

