Kinetic analysis of the guanine nucleotide exchange activity of TRAPP, a multimeric Ypt1p exchange factor
- PMID: 19361519
- PMCID: PMC2770256
- DOI: 10.1016/j.jmb.2009.03.068
Kinetic analysis of the guanine nucleotide exchange activity of TRAPP, a multimeric Ypt1p exchange factor
Abstract
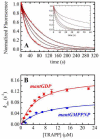
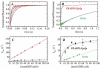
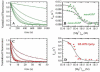
TRAPP complexes, which are large multimeric assemblies that function in membrane traffic, are guanine nucleotide exchange factors (GEFs) that activate the Rab GTPase Ypt1p. Here we measured rate and equilibrium constants that define the interaction of Ypt1p with guanine nucleotide (guanosine 5'-diphosphate and guanosine 5'-triphosphate/guanosine 5'-(beta,gamma-imido)triphosphate) and the core TRAPP subunits required for GEF activity. These parameters allowed us to identify the kinetic and thermodynamic bases by which TRAPP catalyzes nucleotide exchange from Ypt1p. Nucleotide dissociation from Ypt1p is slow (approximately 10(-4) s(-1)) and accelerated >1000-fold by TRAPP. Acceleration of nucleotide exchange by TRAPP occurs via a predominantly Mg(2+)-independent pathway. Thermodynamic linkage analysis indicates that TRAPP weakens nucleotide affinity by <80-fold and vice versa, in contrast to most other characterized GEF systems that weaken nucleotide binding affinities by 4-6 orders of magnitude. The overall net changes in nucleotide binding affinities are small because TRAPP accelerates both nucleotide binding and dissociation from Ypt1p. Weak thermodynamic coupling allows TRAPP, Ypt1p, and nucleotide to exist as a stable ternary complex, analogous to strain-sensing cytoskeleton motors. These results illustrate a novel strategy of guanine nucleotide exchange by TRAPP that is particularly suited for a multifunctional GEF involved in membrane traffic.
Figures
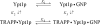
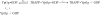
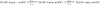
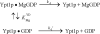
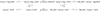
References
-
- Bourne HR, Sanders DA, McCormick F. The GTPase superfamily: a conserved switch for diverse cell functions. Nature. 1990;348:125–32. - PubMed
-
- Goody RS, Hofmann-Goody W. Exchange factors, effectors, GAPs and motor proteins: common thermodynamic and kinetic principles for different functions. Eur Biophys J. 2002;31:268–74. - PubMed
-
- Cai H, Reinisch K, Ferro-Novick S. Coats, tethers, Rabs, and SNAREs work together to mediate the intracellular destination of a transport vesicle. Dev Cell. 2007;12:671–82. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

