Fludarabine-melphalan conditioning for AML and MDS: alemtuzumab reduces acute and chronic GVHD without affecting long-term outcomes
- PMID: 19361753
- PMCID: PMC4348112
- DOI: 10.1016/j.bbmt.2009.01.021
Fludarabine-melphalan conditioning for AML and MDS: alemtuzumab reduces acute and chronic GVHD without affecting long-term outcomes
Abstract
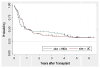
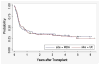
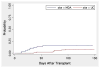
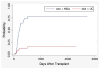
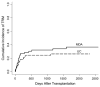
The purpose of this study was to determine the effect of alemtuzumab on treatment-related mortality (TRM), relapse, overall survival (OS), and disease-free survival (DSF) in patients with acute myelogenous leukemia (AML) and myelodysplastic syndromes (MDS) undergoing reduced intensity conditioning (RIC). We compared the outcome of 95 patients treated at the University of Chicago with fludarabine melphalan (Flu + Mel) + alemtuzumab conditioning and 59 patients treated at the M.D. Anderson Cancer Center with Flu + Mel conditioning. Both groups had similar patient and donor characteristics. There were no significant differences in TRM, relapse, survival, and DFS between the 2 groups. The incidence of acute graft-versus-host disease (aGVHD) grade II-IV (relative risk [RR] 5.5, P < .01) and chronic GVHD (cGVHD) (RR 6.6, P < .01) were significantly lower in patients receiving alemtuzumab. The addition of alemtuzumab to an RIC regimen dramatically reduces the incidence of aGVHD and cGVHD in patients with AML and MDS undergoing allogeneic transplantation. TRM, relapse risk, OS and DFS are not affected.
Figures
References
-
- van Besien K, Artz A, Stock W. Unrelated donor transplantation over the age of 55. Are we merely getting (b)older? Leukemia. 2005;19:31–33. - PubMed
-
- Shimoni A, Kroger N, Zabelina T, et al. Hematopoietic stem-cell transplantation from unrelated donors in elderly patients (age >55 years) with hematologic malignancies: older age is no longer a contraindication when using reduced intensity conditioning. Leukemia. 2005;19:7–12. - PubMed
-
- Wong R, Giralt SA, Martin T, et al. Reduced-intensity conditioning for unrelated donor hematopoietic stem cell transplantation as treatment for myeloid malignancies in patients older than 55 years. Blood. 2003;102:3052–3059. - PubMed
-
- de Lima M, Anagnostopoulos A, Munsell M, et al. Non-ablative versus reduced intensity conditioning regimens in the treatment of acute myeloid leukemia and high-risk myelodysplastic syndrome. Dose is relevant for long-term disease control after allogeneic hematopoietic stem cell transplantation. Blood. 2004;104:865–872. - PubMed
-
- Giralt S, Cohen R, Mehra R, et al. Preliminary results of fludarabine/melphalan or 2CDA/melphalan as preparative regimens for allogeneic progenitor cell transplantation in poor candidates for myeloablative conditioning [abstract] Blood. 1997;90(Suppl 1):1853.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous