Engineering retinal progenitor cell and scrollable poly(glycerol-sebacate) composites for expansion and subretinal transplantation
- PMID: 19361860
- PMCID: PMC4109162
- DOI: 10.1016/j.biomaterials.2009.02.046
Engineering retinal progenitor cell and scrollable poly(glycerol-sebacate) composites for expansion and subretinal transplantation
Abstract
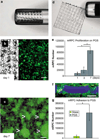
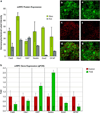
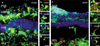
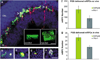
Retinal degenerations cause permanent visual loss and affect millions world-wide. Presently, a novel treatment highlights the potential of using biodegradable polymer scaffolds to induce differentiation and deliver retinal progenitor cells for cell replacement therapy. In this study, we engineered and analyzed a micro-fabricated polymer, poly(glycerol sebacate) (PGS) scaffold, whose useful properties include biocompatibility, elasticity, porosity, and a microtopology conducive to mouse retinal progenitor cell (mRPC) differentiation. In vitro proliferation assays revealed that PGS held up to 86,610 (+/-9993) mRPCs per square millimeter, which were retained through simulated transplantations. mRPCs adherent to PGS differentiated toward mature phenotypes as evidenced by changes in mRNA, protein levels, and enhanced sensitivity to glutamate. Transplanted composites demonstrated long-term mRPC survival and migrated cells exhibited mature marker expression in host retina. These results suggest that combining mRPCs with PGS scaffolds for subretinal transplantation is a practical strategy for advancing retinal tissue engineering as a restorative therapy.
Figures



References
-
- Margalit E, Sadda SR. Retinal and optic nerve diseases. Artif Organs. 2003;27(11):963–974. - PubMed
-
- Klassen HJ, Ng TF, Kurimoto Y, Kirov I, Shatos M, Coffey P, et al. Multipotent retinal progenitors express developmental markers, differentiate into neurons, and preserve light-mediated behavior. Invest Ophthalmol Vis Sci. 2004;45(11):4167–4173. - PubMed
-
- MacLaren RE, Pearson RA, MacNeil A, Douglas RH, Salt TE, Akimoto M, et al. Retinal repair by transplantation of photoreceptor precursors. Nature. 2006;444(7116):203–207. - PubMed
-
- Tomita M, Lavik E, Klassen H, Zahir T, Langer R, Young MJ. Biodegradable polymer composite grafts promote the survival and differentiation of retinal progenitor cells. Stem Cells. 2005;23(10):1579–1588. - PubMed
-
- Tao S, Young C, Redenti S, Zhang Y, Klassen H, Desai T, et al. Survival, migration and differentiation of retinal progenitor cells transplanted on micro-machined poly(methyl methacrylate) scaffolds to the subretinal space. Lab Chip. 2007;7(6):695–701. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

