Lyn kinase controls basophil GATA-3 transcription factor expression and induction of Th2 cell differentiation
- PMID: 19362019
- PMCID: PMC2772996
- DOI: 10.1016/j.immuni.2009.02.008
Lyn kinase controls basophil GATA-3 transcription factor expression and induction of Th2 cell differentiation
Abstract
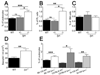
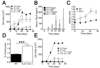
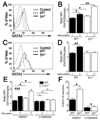
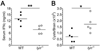
T helper 1 (Th1)-Th2 cell balance is key to host defense and its dysregulation has pathophysiological consequences. Basophils are important in Th2 cell differentiation. However, the factors controlling the onset and extent of basophil-mediated Th2 cell differentiation are unknown. Here, we demonstrate that Lyn kinase dampened basophil expression of the transcription factor GATA-3 and the initiation and extent of Th2 cell differentiation. Lyn-deficient mice had a marked basophilia, a constitutive Th2 cell skewing that was exacerbated upon in vivo challenge of basophils, produced antibodies to a normally inert antigen, and failed to appropriately respond to a Th1 cell-inducing pathogen. The Th2 cell skewing was dependent on basophils, immunoglobulin E, and interleukin-4, but was independent of mast cells. Our findings demonstrate that basophil-expressed Lyn kinase exerts regulatory control on Th2 cell differentiation and function.
Figures



Similar articles
-
Negative control of basophil expansion by IRF-2 critical for the regulation of Th1/Th2 balance.Blood. 2005 Sep 15;106(6):2011-7. doi: 10.1182/blood-2005-04-1344. Epub 2005 May 24. Blood. 2005. PMID: 15914553
-
Induction of Th2 type immunity in a mouse system reveals a novel immunoregulatory role of basophils.Blood. 2007 Apr 1;109(7):2921-7. doi: 10.1182/blood-2006-07-037739. Blood. 2007. PMID: 17132717
-
SHIP represses Th2 skewing by inhibiting IL-4 production from basophils.J Immunol. 2011 Jan 1;186(1):323-32. doi: 10.4049/jimmunol.1002778. Epub 2010 Dec 3. J Immunol. 2011. PMID: 21131429
-
Recent developments in basophil research: do basophils initiate and perpetuate type 2 T-helper cell responses?Int Arch Allergy Immunol. 2013;160(1):7-17. doi: 10.1159/000341633. Epub 2012 Aug 30. Int Arch Allergy Immunol. 2013. PMID: 22948001 Review.
-
'All things considered': transcriptional regulation of T helper type 2 cell differentiation from precursor to effector activation.Immunology. 2013 Sep;140(1):31-8. doi: 10.1111/imm.12121. Immunology. 2013. PMID: 23668241 Free PMC article. Review.
Cited by
-
Sphingosine-1-phosphate can promote mast cell hyper-reactivity through regulation of contactin-4 expression.J Leukoc Biol. 2013 Nov;94(5):1013-24. doi: 10.1189/jlb.0313163. Epub 2013 Jul 31. J Leukoc Biol. 2013. PMID: 23904439 Free PMC article.
-
Basophils and the T helper 2 environment can promote the development of lupus nephritis.Nat Med. 2010 Jun;16(6):701-7. doi: 10.1038/nm.2159. Epub 2010 May 30. Nat Med. 2010. PMID: 20512127 Free PMC article.
-
Cutting edge: persistence of increased mast cell numbers in tissues links dermatitis to enhanced airway disease in a mouse model of atopy.J Immunol. 2012 Jan 15;188(2):531-5. doi: 10.4049/jimmunol.1102703. Epub 2011 Dec 16. J Immunol. 2012. PMID: 22180615 Free PMC article.
-
Basophils and Systemic Lupus Erythematosus in Murine Models and Human Patients.Biology (Basel). 2020 Sep 23;9(10):308. doi: 10.3390/biology9100308. Biology (Basel). 2020. PMID: 32977704 Free PMC article. Review.
-
AMG853, A Bispecific Prostaglandin D2 Receptor 1 and 2 Antagonist, Dampens Basophil Activation and Related Lupus-Like Nephritis Activity in Lyn-Deficient Mice.Front Immunol. 2022 Apr 4;13:824686. doi: 10.3389/fimmu.2022.824686. eCollection 2022. Front Immunol. 2022. PMID: 35444641 Free PMC article.
References
-
- Beavitt SJ, Harder KW, Kemp JM, Jones J, Quilici C, Casagranda F, Lam E, Turner D, Brennan S, Sly PD. Lyn-deficient mice develop severe, persistent asthma: Lyn is a critical negative regulator of Th2 immunity. J. Immunol. 2005;175:1867–1875. - PubMed
-
- Cannons JL, Yu LJ, Hill B, Mijares LA, Dombroski D, Nichols KE, Antonellis A, Koretzky GA, Gardner K, Schwartzberg PL. SAP regulates T(H)2 differentiation and PKC-theta-mediated activation of NF-κB1. Immunity. 2004;21:693–706. - PubMed
-
- Capron A, Dombrowicz D, Capron M. Helminth infections and allergic diseases: from the Th2 paradigm to regulatory networks. Clin. Rev. Allergy Immunol. 2004;26:25–34. - PubMed
-
- Davidson D, Shi X, Zhang S, Wang H, Nemer M, Ono N, Ohno S, Yanagi Y, Veillette A. Genetic evidence linking SAP, the X-linked lymphoproliferative gene product, to Src-related kinase FynT in T(H)2 cytokine regulation. Immunity. 2004;21:707–717. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

