Molecular Interactions between HIV-1 integrase and the two viral DNA ends within the synaptic complex that mediates concerted integration
- PMID: 19362096
- PMCID: PMC2791363
- DOI: 10.1016/j.jmb.2009.04.007
Molecular Interactions between HIV-1 integrase and the two viral DNA ends within the synaptic complex that mediates concerted integration
Abstract
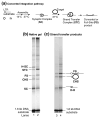
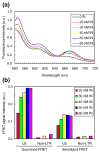
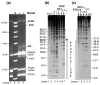
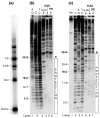
A macromolecular nucleoprotein complex in retrovirus-infected cells, termed the preintegration complex, is responsible for the concerted integration of linear viral DNA genome into host chromosomes. Isolation of sufficient quantities of the cytoplasmic preintegration complexes for biochemical and biophysical analysis is difficult. We investigated the architecture of HIV-1 nucleoprotein complexes involved in the concerted integration pathway in vitro. HIV-1 integrase (IN) non-covalently juxtaposes two viral DNA termini forming the synaptic complex, a transient intermediate in the integration pathway, and shares properties associated with the preintegration complex. IN slowly processes two nucleotides from the 3' OH ends and performs the concerted insertion of two viral DNA ends into target DNA. IN remains associated with the concerted integration product, termed the strand transfer complex. The synaptic complex and strand transfer complex can be isolated by native agarose gel electrophoresis. In-gel fluorescence resonance energy transfer measurements demonstrated that the energy transfer efficiencies between the juxtaposed Cy3 and Cy5 5'-end labeled viral DNA ends in the synaptic complex (0.68+/-0.09) was significantly different from that observed in the strand transfer complex (0.07+/-0.02). The calculated distances were 46+/-3 A and 83+/-5 A, respectively. DNaseI footprint analysis of the complexes revealed that IN protects U5 and U3 DNA sequences up to approximately 32 bp from the end, suggesting two IN dimers were bound per terminus. Enhanced DNaseI cleavages were observed at nucleotide positions 6 and 9 from the terminus on U3 but not on U5, suggesting independent assembly events. Protein-protein cross-linking of IN within these complexes revealed the presence of dimers, tetramers, and a larger multimer (>120 kDa). Our results suggest a new model where two IN dimers individually assemble on U3 and U5 ends before the non-covalent juxtaposition of two viral DNA ends, producing the synaptic complex.
Figures





References
-
- Brown PO. Integration. In: Coffin JM, Hughes S, Varmus H, editors. Retroviruses. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1997. pp. 161–203. - PubMed
-
- Chen H, Wei SQ, Engelman A. Multiple integrase functions are required to form the native structure of the human immunodeficiency virus type I intasome. J Biol Chem. 1999;274:17358–17364. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

