Permeability of the blood-brain barrier depends on brain temperature
- PMID: 19362131
- PMCID: PMC2694729
- DOI: 10.1016/j.neuroscience.2009.04.004
Permeability of the blood-brain barrier depends on brain temperature
Abstract
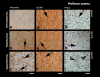
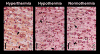
Increased permeability of the blood-brain barrier (BBB) has been reported in different conditions accompanied by hyperthermia, but the role of brain temperature per se in modulating brain barrier functions has not been directly examined. To delineate the contribution of this factor, we examined albumin immunoreactivity in several brain structures (cortex, hippocampus, thalamus and hypothalamus) of pentobarbital-anesthetized rats (50 mg/kg i.p.), which were passively warmed to different levels of brain temperature (32-42 degrees C). Similar brain structures were also examined for the expression of glial fibrillary acidic protein (GFAP), an index of astrocytic activation, water and ion content, and morphological cell abnormalities. Data were compared with those obtained from drug-free awake rats with normal brain temperatures (36-37 degrees C). The numbers of albumin- and GFAP-positive cells strongly correlate with brain temperature, gradually increasing from approximately 38.5 degrees C and plateauing at 41-42 degrees C. Brains maintained at hyperthermia also showed larger content of brain water and Na(+), K(+) and Cl(-) as well as structural abnormalities of brain cells, all suggesting acute brain edema. The latter alterations were seen at approximately 39 degrees C, gradually progressed with temperature increase, and peaked at maximum hyperthermia. Temperature-dependent changes in albumin immunoreactivity tightly correlated with GFAP immunoreactivity, brain water, and numbers of abnormal cells; they were found in each tested area, but showed some structural specificity. Notably, a mild BBB leakage, selective glial activation, and specific cellular abnormalities were also found in the hypothalamus and piriform cortex during extreme hypothermia (32-33 degrees C); in contrast to hyperthermia these changes were associated with decreased levels of brain water, Na(+) and K(+), suggesting acute brain dehydration. Therefore, brain temperature per se is an important factor in regulating BBB permeability, alterations in brain water homeostasis, and subsequent structural abnormalities of brain cells.
Figures






References
-
- Arican N, Kaya M, Yorulmaz C, Kalayci R, Ince H, Kucuk M, Fincanci SK, Elmas I. Effect of hypothermia on blood-brain barrier permeability following traumatic brain injury in chronically treated rats. Int J Neurosci. 2006;116:1249–126. - PubMed
-
- Bechtold DA, Brown IR. Induction of Hsp27 and Hsp32 stress proteins and vimentin in glial cells of the rat hippocampus following hyperthermia. Neurochem Res. 2003;28:1163–1173. - PubMed
-
- Bekey L, Lee JC, Lee GC, Peng GR. Experimental cerebral concussion: an electron microscopic study. J Neurosurg. 1977;47:525–531. - PubMed
-
- Bondarenko A, Chesler M. Rapid astrocyte death induced by transient hypoxia, acidosis, and extracellular ion shifts. Glia. 2001;34:134–142. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

