Discovery of thioether-bridged cyclic pentapeptides binding to Grb2-SH2 domain with high affinity
- PMID: 19362470
- PMCID: PMC7291730
- DOI: 10.1016/j.bmcl.2009.03.134
Discovery of thioether-bridged cyclic pentapeptides binding to Grb2-SH2 domain with high affinity
Abstract
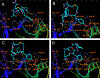
Blocking the interaction between phosphotyrosine (pTyr)-containing activated receptors and the Src homology 2 (SH2) domain of the growth factor receptor-bound protein 2 (Grb 2) is considered to be an effective and non-cytotoxic strategy to develop new anti-proliferate agents due to its potential to shut down the Ras activation pathway. In this study, a series of phosphotyrosine containing cyclic pentapeptides were designed and synthesized based upon the phage library derived cyclopeptide, G1TE. A comprehensive SAR study was also carried out to develop potent Grb2-SH2 domain antagonists based upon this novel template. With both the peptidomimetic optimization of the amino acid side-chains and the constraint of the backbone conformation guided by molecular modeling, we developed several potent antagonists with low micromolar range binding affinity, such as cyclic peptide 15 with an K(d)=0.359microM, which is providing a novel template for the development of Grb2-SH2 domain antagonists as potential therapeutics for certain cancers.
Figures

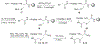
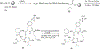

Similar articles
-
Structural basis for a non-phosphorus-containing cyclic peptide binding to Grb2-SH2 domain with high affinity.Biochem Biophys Res Commun. 2003 Aug 8;307(4):1038-44. doi: 10.1016/s0006-291x(03)01291-9. Biochem Biophys Res Commun. 2003. PMID: 12878216
-
Structure-based design of potent Grb2-SH2 domain antagonists not relying on phosphotyrosine mimics.Biochem Biophys Res Commun. 2006 Oct 20;349(2):497-503. doi: 10.1016/j.bbrc.2006.08.059. Epub 2006 Aug 22. Biochem Biophys Res Commun. 2006. PMID: 16945340
-
Discovery of a novel nonphosphorylated pentapeptide motif displaying high affinity for Grb2-SH2 domain by the utilization of 3'-substituted tyrosine derivatives.J Med Chem. 2006 Mar 9;49(5):1585-96. doi: 10.1021/jm050910x. J Med Chem. 2006. PMID: 16509576
-
Grb2 SH2 domain-binding peptide analogs as potential anticancer agents.Biopolymers. 2003;71(2):132-40. doi: 10.1002/bip.10396. Biopolymers. 2003. PMID: 12767115 Review.
-
The Role of Grb2 in Cancer and Peptides as Grb2 Antagonists.Protein Pept Lett. 2018 Feb 8;24(12):1084-1095. doi: 10.2174/0929866525666171123213148. Protein Pept Lett. 2018. PMID: 29173143 Review.
Cited by
-
An improved synthesis of Compound 11, a unique bicyclic melanocortin-3 antagonist.Pept Sci (Hoboken). 2025 Jan;117(1):e24385. doi: 10.1002/pep2.24385. Epub 2024 Nov 22. Pept Sci (Hoboken). 2025. PMID: 40452822
-
Targeting kinase signaling pathways with constrained peptide scaffolds.Pharmacol Ther. 2017 May;173:159-170. doi: 10.1016/j.pharmthera.2017.02.014. Epub 2017 Feb 7. Pharmacol Ther. 2017. PMID: 28185915 Free PMC article. Review.
-
A comparative study of short linear motif compositions of the influenza A virus ribonucleoproteins.PLoS One. 2012;7(6):e38637. doi: 10.1371/journal.pone.0038637. Epub 2012 Jun 8. PLoS One. 2012. PMID: 22715401 Free PMC article.
-
Enantioselective Transesterification Catalysis by Nanosized Serine Protease Subtilisin Carlsberg Particles in Tetrahydrofuran.Tetrahedron. 2010 Mar 20;66(12):2175-2180. doi: 10.1016/j.tet.2010.01.053. Tetrahedron. 2010. PMID: 20661313 Free PMC article.
References
-
- Margolis B Prog. Biophys. Mol. Biol 1994, 62, 223. - PubMed
-
- Yip SS; Crew AJ; Gee JM; Hui R; Blamey RW; Robertson JF; Nicholson RI; Sutherland RL; Daly RJ Int. J. Cancer 2000, 88, 363. - PubMed
-
- Lim SJ; Lopez-Berestein G; Hung MC; Lupu R; Tari AM Oncogene 2000, 19, 6271. - PubMed
-
- Caravatti G; Rahuel J; Gay B; Furet P Bioorg. Med. Chem. Lett 1999, 9, 1973. - PubMed
-
- Long Y-Q; Yao Z-J; Voigt JH; Lung F-DT; Luo JH; Burke TR; King CR; Yang D; Roller PP Biochem. Biophys. Res. Commun 1999, 264, 902. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Research Materials
Miscellaneous

