Metabolic defects provide a spark for the epigenetic switch in cancer
- PMID: 19362589
- PMCID: PMC2728018
- DOI: 10.1016/j.freeradbiomed.2009.04.010
Metabolic defects provide a spark for the epigenetic switch in cancer
Abstract
Cancer is a pathology that is associated with aberrant gene expression and an altered metabolism. Whereas changes in gene expression have historically been attributed to mutations, it has become apparent that epigenetic processes also play a critical role in controlling gene expression during carcinogenesis. Global changes in epigenetic processes, including DNA methylation and histone modifications, have been observed in cancer. These epigenetic alterations can aberrantly silence or activate gene expression during the formation of cancer; however, the process leading to this epigenetic switch in cancer remains unknown. Carcinogenesis is also associated with metabolic defects that increase mitochondrially derived reactive oxygen species, create an atypical redox state, and change the fundamental means by which cells produce energy. Here, we summarize the influence of these metabolic defects on epigenetic processes. Metabolic defects affect epigenetic enzymes by limiting the availability of cofactors like S-adenosylmethionine. Increased production of reactive oxygen species alters DNA methylation and histone modifications in tumor cells by oxidizing DNMTs and HMTs or through direct oxidation of nucleotide bases. Last, the Warburg effect and increased glutamine consumption in cancer influence histone acetylation and methylation by affecting the activity of sirtuins and histone demethylases.
Figures
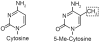





Similar articles
-
An Update of Epigenetic Drugs for the Treatment of Cancers and Brain Diseases: A Comprehensive Review.Genes (Basel). 2023 Apr 6;14(4):873. doi: 10.3390/genes14040873. Genes (Basel). 2023. PMID: 37107631 Free PMC article. Review.
-
Role of glutathione in the regulation of epigenetic mechanisms in disease.Free Radic Biol Med. 2017 Nov;112:36-48. doi: 10.1016/j.freeradbiomed.2017.07.008. Epub 2017 Jul 10. Free Radic Biol Med. 2017. PMID: 28705657 Review.
-
Redox regulation of the epigenetic landscape in cancer: a role for metabolic reprogramming in remodeling the epigenome.Free Radic Biol Med. 2012 Dec 1;53(11):2178-87. doi: 10.1016/j.freeradbiomed.2012.09.028. Epub 2012 Sep 26. Free Radic Biol Med. 2012. PMID: 23022407 Free PMC article. Review.
-
An epigenetic perspective on the free radical theory of development.Free Radic Biol Med. 2007 Oct 1;43(7):1023-36. doi: 10.1016/j.freeradbiomed.2007.06.027. Epub 2007 Jul 10. Free Radic Biol Med. 2007. PMID: 17761298 Free PMC article. Review.
-
DNA methylation, histone acetylation and methylation of epigenetic modifications as a therapeutic approach for cancers.Cancer Lett. 2016 Apr 10;373(2):185-92. doi: 10.1016/j.canlet.2016.01.036. Epub 2016 Jan 22. Cancer Lett. 2016. PMID: 26808576 Review.
Cited by
-
The Warburg effect: molecular aspects and therapeutic possibilities.Mol Biol Rep. 2015 Apr;42(4):825-34. doi: 10.1007/s11033-014-3764-7. Mol Biol Rep. 2015. PMID: 25253100 Review.
-
Zebrafish as an In Vivo Model to Assess Epigenetic Effects of Ionizing Radiation.Int J Mol Sci. 2016 Dec 15;17(12):2108. doi: 10.3390/ijms17122108. Int J Mol Sci. 2016. PMID: 27983682 Free PMC article. Review.
-
From inflammaging to healthy aging by dietary lifestyle choices: is epigenetics the key to personalized nutrition?Clin Epigenetics. 2015 Mar 25;7(1):33. doi: 10.1186/s13148-015-0068-2. eCollection 2015. Clin Epigenetics. 2015. PMID: 25861393 Free PMC article.
-
Prolonged exposure to particulate pollution, genes associated with glutathione pathways, and DNA methylation in a cohort of older men.Environ Health Perspect. 2011 Jul;119(7):977-82. doi: 10.1289/ehp.1002773. Epub 2011 Mar 8. Environ Health Perspect. 2011. PMID: 21385671 Free PMC article.
-
Oxygen Regulates Human Pluripotent Stem Cell Metabolic Flux.Stem Cells Int. 2019 May 19;2019:8195614. doi: 10.1155/2019/8195614. eCollection 2019. Stem Cells Int. 2019. PMID: 31236115 Free PMC article.
References
-
- Oberley LW, Buettner GR. Role of superoxide dismutase in cancer: a review. Cancer Res. 1979;39:1141–1149. - PubMed
-
- Oberley LW, Oberley TD, Buettner GR. Cell division in normal and transformed cells: the possible role of superoxide and hydrogen peroxide. Med Hypotheses. 1981;7:21–42. - PubMed
-
- Gius D, Spitz DR. Redox signaling in cancer biology. Antioxidants & redox signaling. 2006;8:1249–1252. - PubMed
-
- Spitz DR, Sim JE, Ridnour LA, Galoforo SS, Lee YJ. Glucose deprivation-induced oxidative stress in human tumor cells. A fundamental defect in metabolism? Annals of the New York Academy of Sciences. 2000;899:349–362. - PubMed
-
- Cerutti PA. Prooxidant states and tumor promotion. Science. 1985;227:375–381. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

