Bacterial fatty acid synthesis and its relationships with polyketide synthetic pathways
- PMID: 19362649
- PMCID: PMC4095770
- DOI: 10.1016/S0076-6879(09)04617-5
Bacterial fatty acid synthesis and its relationships with polyketide synthetic pathways
Abstract
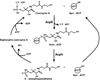
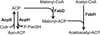
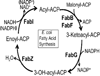
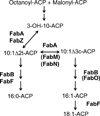
This review presents the most thoroughly studied bacterial fatty acid synthetic pathway, that of Escherichia coli and then discusses the exceptions to the E. coli pathway present in other bacteria. The known interrelationships between the fatty acid and polyketide synthetic pathways are also assessed, mainly in the Streptomyces group of bacteria. Finally, we present a compendium of methods for analysis of bacterial fatty acid synthetic pathways.
Figures

References
-
- Alberts AA, Majerus PW, Vagelos PR. Malonyl-CoA acyl carrier protein transacylase. Meth. Enzymol. 1974;14:53–56.
-
- Alberts AW, Bell RM, Vagelos PR. Acyl carrier protein. XV. Studies of β-ketoacyl-acyl carrier protein synthetase. J. Biol. Chem. 1972;247:3190–3198. - PubMed
-
- Arthur CJ, Szafranska A, Evans SE, Findlow SC, Burston SG, Owen P, Clark-Lewis I, Simpson TJ, Crosby J, Crump MP. Self-malonylation is an intrinsic property of a chemically synthesized type II polyketide synthase acyl carrier protein. Biochemistry. 2005;44:15414–15421. - PubMed
-
- Arthur CJ, Szafranska AE, Long J, Mills J, Cox RJ, Findlow SC, Simpson TJ, Crump MP, Crosby J. The malonyl transferase activity of type II polyketide synthase acyl carrier proteins. Chem. Biol. 2006;13:587–596. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

