Impact of mailed and automated telephone reminders on receipt of repeat mammograms: a randomized controlled trial
- PMID: 19362800
- PMCID: PMC2698939
- DOI: 10.1016/j.amepre.2009.01.032
Impact of mailed and automated telephone reminders on receipt of repeat mammograms: a randomized controlled trial
Abstract
Background: This study compares the efficacy of three types of reminders in promoting annual repeat mammography screening.
Design: RCT.
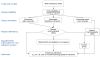
Setting and participants: Study recruitment occurred in 2004-2005. Participants were recruited through the North Carolina State Health Plan for Teachers and State Employees. All were aged 40-75 years and had a screening mammogram prior to study enrollment. A total of 3547 women completed baseline telephone interviews.
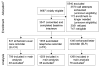
Intervention: Prior to study recruitment, women were assigned randomly to one of three reminder groups: (1) printed enhanced usual care reminders (EUCRs); (2) automated telephone reminders (ATRs) identical in content to EUCRs; or (3) enhanced letter reminders that included additional information guided by behavioral theory. Interventions were delivered 2-3 months prior to women's mammography due dates.
Main outcome measures: Repeat mammography adherence, defined as having a mammogram no sooner than 10 months and no later than 14 months after the enrollment mammogram.
Results: Each intervention produced adherence proportions that ranged from 72% to 76%. Post-intervention adherence rates increased by an absolute 17.8% from baseline. Women assigned to ATRs were significantly more likely to have had mammograms than women assigned to EUCRs (p=0.014). Comparisons of reminder efficacy did not vary across key subgroups.
Conclusions: Although all reminders were effective in promoting repeat mammography adherence, ATRs were the most effective and lowest in cost. Health organizations should consider using ATRs to maximize proportions of members who receive mammograms at annual intervals.
Figures
Similar articles
-
Finding the minimal intervention needed for sustained mammography adherence.Am J Prev Med. 2010 Oct;39(4):334-44. doi: 10.1016/j.amepre.2010.05.020. Am J Prev Med. 2010. PMID: 20837284 Free PMC article. Clinical Trial.
-
Implementation and process evaluation of three interventions to promote screening mammograms delivered for 4 years in a large primary care population.Transl Behav Med. 2017 Sep;7(3):547-556. doi: 10.1007/s13142-017-0497-x. Transl Behav Med. 2017. PMID: 28452044 Free PMC article. Clinical Trial.
-
Effect of a multimodal reminder program on repeat mammogram screening.Am J Prev Med. 2009 Aug;37(2):94-101. doi: 10.1016/j.amepre.2009.03.022. Am J Prev Med. 2009. PMID: 19589447 Free PMC article.
-
Testing reminder and motivational telephone calls to increase screening mammography: a randomized study.J Natl Cancer Inst. 2000 Feb 2;92(3):233-42. doi: 10.1093/jnci/92.3.233. J Natl Cancer Inst. 2000. PMID: 10655440 Clinical Trial.
-
Manually-generated reminders delivered on paper: effects on professional practice and patient outcomes.Cochrane Database Syst Rev. 2019 Dec 18;12(12):CD001174. doi: 10.1002/14651858.CD001174.pub4. Cochrane Database Syst Rev. 2019. PMID: 31858588 Free PMC article.
Cited by
-
Economics of Multicomponent Interventions to Increase Breast, Cervical, and Colorectal Cancer Screening: A Community Guide Systematic Review.Am J Prev Med. 2019 Oct;57(4):557-567. doi: 10.1016/j.amepre.2019.03.006. Epub 2019 Aug 30. Am J Prev Med. 2019. PMID: 31477431 Free PMC article.
-
Sustaining mammography screening among the medically underserved: a follow-up evaluation.J Womens Health (Larchmt). 2015 Apr;24(4):291-8. doi: 10.1089/jwh.2014.4967. Epub 2015 Feb 18. J Womens Health (Larchmt). 2015. PMID: 25692910 Free PMC article.
-
It's the amount of thought that counts: when ambivalence contributes to mammography screening delay.Womens Health Issues. 2012 Mar;22(2):e189-94. doi: 10.1016/j.whi.2011.08.008. Epub 2011 Nov 3. Womens Health Issues. 2012. PMID: 22055988 Free PMC article.
-
Counseling patients on facial volume replacement and adherence with posttreatment instructions.Patient Prefer Adherence. 2010 Sep 7;4:273-81. doi: 10.2147/ppa.s11024. Patient Prefer Adherence. 2010. PMID: 20859454 Free PMC article.
-
What implementation interventions increase cancer screening rates? a systematic review.Implement Sci. 2011 Sep 29;6:111. doi: 10.1186/1748-5908-6-111. Implement Sci. 2011. PMID: 21958556 Free PMC article.
References
-
- Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58(2):71–96. - PubMed
-
- Berry DA, Cronin KA, Plevritis SK, et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med. 2005;353(17):1784–92. - PubMed
-
- Humphrey LL, Helfand M, Chan BK, Woolf SH. Breast cancer screening: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2002;137(5 Pt 1):347–60. - PubMed
-
- Tabar L, Yen MF, Vitak B, Chen HH, Smith RA, Duffy SW. Mammography service screening and mortality in breast cancer patients: 20-year follow-up before and after introduction of screening. Lancet. 2003;361(9367):1405–10. - PubMed
-
- Smith RA, Saslow D, Sawyer KA, et al. American Cancer Society guidelines for breast cancer screening: update 2003. CA Cancer J Clin. 2003;53(3):141–69. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

