Antibiotic resistance in Mycobacterium tuberculosis: peroxidase intermediate bypass causes poor isoniazid activation by the S315G mutant of M. tuberculosis catalase-peroxidase (KatG)
- PMID: 19363028
- PMCID: PMC2713512
- DOI: 10.1074/jbc.M109.005546
Antibiotic resistance in Mycobacterium tuberculosis: peroxidase intermediate bypass causes poor isoniazid activation by the S315G mutant of M. tuberculosis catalase-peroxidase (KatG)
Abstract
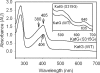
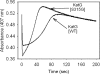
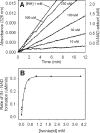
KatG (catalase-peroxidase) in Mycobacterium tuberculosis is responsible for activation of isoniazid (INH), a pro-drug used to treat tuberculosis infections. Resistance to INH is a global health problem most often associated with mutations in the katG gene. The origin of INH resistance caused by the KatG[S315G] mutant enzyme is examined here. Overexpressed KatG[S315G] was characterized by optical, EPR, and resonance Raman spectroscopy and by studies of the INH activation mechanism in vitro. Catalase activity and peroxidase activity with artificial substrates were moderately reduced (50 and 35%, respectively), whereas the rates of formation of oxyferryl heme:porphyrin pi-cation radical and the decay of heme intermediates were approximately 2-fold faster in KatG[S315G] compared with WT enzyme. The INH binding affinity for the resting enzyme was unchanged, whereas INH activation, measured by the rate of formation of an acyl-nicotinamide adenine dinucleotide adduct considered to be a bactericidal molecule, was reduced by 30% compared with WT KatG. INH resistance is suggested to arise from a redirection of catalytic intermediates into nonproductive reactions that interfere with oxidation of INH. In the resting mutant enzyme, a rapid evolution of 5-c heme to 6-c species occurred in contrast with the behavior of WT KatG and KatG[S315T] and consistent with greater flexibility at the heme edge in the absence of the hydroxyl of residue 315. Insights into the effects of mutations at residue 315 on enzyme structure, peroxidation kinetics, and specific interactions with INH are presented.
Figures


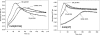
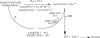

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

