Desaturases: emerging models for understanding functional diversification of diiron-containing enzymes
- PMID: 19363032
- PMCID: PMC2707227
- DOI: 10.1074/jbc.R900009200
Desaturases: emerging models for understanding functional diversification of diiron-containing enzymes
Abstract
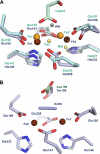
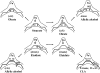
Desaturases and related enzymes perform O(2)-dependent dehydrogenations initiated at unactivated C-H groups with the use of a diiron active site. Determination of the long-sought oxidized desaturase crystal structure facilitated structural comparison of the active sites of disparate diiron enzymes. Experiments on the castor desaturase are discussed that provide experimental support for a hypothesized ancestral oxidase enzyme in the context of the evolution of the diiron enzyme diverse functionality. We also summarize recent analysis of a castor mutant desaturase that provides valuable insights into the relationship of proposed substrate-binding modes with respect to a range of catalytic outcomes.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

