BK channels modulate pre- and postsynaptic signaling at reciprocal synapses in retina
- PMID: 19363492
- PMCID: PMC2704978
- DOI: 10.1038/nn.2302
BK channels modulate pre- and postsynaptic signaling at reciprocal synapses in retina
Abstract
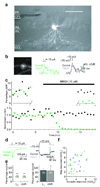
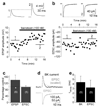
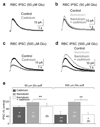
In the mammalian retina, A17 amacrine cells provide reciprocal inhibitory feedback to rod bipolar cells, thereby shaping the time course of visual signaling in vivo. Previous results have indicated that A17 feedback can be triggered by Ca(2+) influx through Ca(2+)-permeable AMPA receptors and can occur independently of voltage-gated Ca(2+) (Ca(v)) channels, whose presence and functional role in A17 dendrites have not yet been explored. We combined electrophysiology, calcium imaging and immunohistochemistry and found that L-type Ca(v) channels in rat A17 amacrine cells were located at the sites of reciprocal synaptic feedback and that their contribution to GABA release was diminished by large-conductance Ca(2+)-activated potassium (BK) channels, which suppress postsynaptic depolarization in A17s and limit Ca(v) channel activation. We also found that BK channels, by limiting GABA release from A17s, regulate the flow of excitatory synaptic transmission through the rod pathway.
Figures





References
-
- Hartveit E. Reciprocal synaptic interactions between rod bipolar cells and amacrine cells in the rat retina. J Neurophysiol. 1999;81:2923–2936. - PubMed
-
- Nelson R, Kolb H. A17: a broad-field amacrine cell in the rod system of the cat retina. J Neurophysiol. 1985;54:592–614. - PubMed
-
- Dong CJ, Hare WA. Temporal modulation of scotopic visual signals by A17 amacrine cells in mammalian retina in vivo. J Neurophysiol. 2003;89:2159–2166. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

