The new face of nucleolin in human melanoma
- PMID: 19363676
- PMCID: PMC11030984
- DOI: 10.1007/s00262-009-0705-8
The new face of nucleolin in human melanoma
Abstract
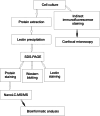
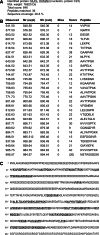
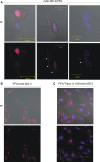
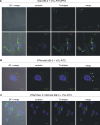
Nucleolin is multifunctional protein mainly present in nucleoli but also detected in cytoplasm and plasma membranes. Extranuclear nucleolin differs from the nuclear form by its glycosylation. Studies on expression of nucleolin in breast cancer suggest a possible association to the metastatic cascade. In the present study, Vicia villosa lectin (VVL) precipitation followed by subsequent polyacrylamide gel electrophoresis and mass spectrometry analysis demonstrates nucleolin as a VVL-positive glycoprotein expressed in melanoma. The presence of VVL-positive nucleolin in the melanoma cell membrane and cytoplasm was confirmed by confocal microscopy. Using bioinformatic peptide prediction programs, nucleolin was shown to contain multiple possible MHC class-I binding peptides in its sequence which makes nucleolin an interesting melanoma marker and target for immunodiagnostic and possibly therapeutic purposes.
Figures


References
-
- Bubenik J. MHC class I down-regulation, tumour escape from immune surveillance and design of therapeutic strategies. Folia Biol (Praha) 2005;51:1–2. - PubMed
-
- Chen C, Chiang S, Yeh N. Increased stability of nucleolin in proliferating cells by inhibition of its self-cleaving activity. J Biol Chem. 1991;266:7754–7758. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

